GlycoRNA: Unveiling a New Frontier in RNA Biology and Its Biomedical Applications
This article provides a comprehensive overview of glycoRNA, a recently discovered class of small non-coding RNAs modified with glycans.
GlycoRNA: Unveiling a New Frontier in RNA Biology and Its Biomedical Applications
Abstract
This article provides a comprehensive overview of glycoRNA, a recently discovered class of small non-coding RNAs modified with glycans. Aimed at researchers, scientists, and drug development professionals, it explores the foundational discovery and biology of glycoRNAs, details cutting-edge detection methodologies and their applications in disease diagnostics and therapeutics, discusses current challenges and optimization strategies in the field, and validates their significance through comparative analysis with other biomolecules. The synthesis of this emerging field highlights the transformative potential of glycoRNAs in immune regulation, cancer biology, and the development of novel biomarkers and targeted therapies.
Discovering GlycoRNA: Redefining the Central Dogma of Molecular Biology
The recent discovery of glycosylated RNA (glycoRNA) represents a fundamental paradigm shift in molecular biology. Traditionally, glycosylation was considered an exclusive modification of proteins and lipids. This review details the breakthrough findings that have identified RNA as a third scaffold for glycosylation, a novel class of biomolecules that are abundantly present on cell surfaces and play critical roles in cellular communication, immune regulation, and disease pathogenesis. We provide an in-depth technical examination of glycoRNA biology, including its composition, biosynthetic pathways, detection methodologies, and functional significance, with a particular emphasis on its implications for cancer biology and therapeutic development. Structured data presentations and experimental workflows are included to serve as a comprehensive resource for researchers and drug development professionals navigating this emerging frontier.
The cell surface serves as the primary interface for interactions between cells and their external environment, playing a critical role in biological regulation through molecular mechanisms including ligand recognition, signal transduction, and cascade initiation [1]. For decades, glycosylation—the enzymatic process of attaching carbohydrates to target molecules—was believed to occur exclusively on proteins and lipids. Extensive research established glycoproteins and glycolipids as central players in diverse biological processes, with clinically relevant tumor biomarkers such as CA19-9 and PSA being widely employed in cancer screening and diagnosis [1].
The discovery that RNA can also serve as a substrate for glycosylation fundamentally challenges this conventional understanding [1]. These glycosylated RNAs (glycoRNAs) represent a previously unrecognized modification that bridges two traditionally separate fields: RNA biology, primarily confined to the nucleus and cytoplasm, and glycobiology, localized to the endoplasmic reticulum-Golgi system [1]. This unexpected convergence has revealed a new layer of complexity in molecular interactions within and outside the cell, opening transformative avenues for research and therapeutic development.
The Fundamental Nature of GlycoRNA
Biochemical Composition and Characteristics
GlycoRNAs are defined as small non-coding RNAs modified with N-glycan structures rich in sialic acid and fucose components [1]. These molecules have been confirmed to exist on the cell surface, suggesting their potential involvement in intercellular communication and immune recognition processes [1]. The unique feature of glycoRNAs lies in their dual composition: they contain specific RNA sequences complexed with sophisticated carbohydrate structures.
Table 1: Primary RNA Species Identified as GlycoRNA Scaffolds
| RNA Type | Full Name | Key Characteristics | References |
|---|---|---|---|
| snRNAs | Small Nuclear RNAs | U2 and U4 are particularly abundant in glioma cells | [1] [2] |
| Y RNAs | - | Y5 identified as a glycosylated species | [2] |
| tRNAs | Transfer RNAs | acp3U modification serves as key anchoring site | [1] [3] |
| snoRNAs | Small Nucleolar RNAs | - | [1] [3] |
| miRNAs | MicroRNAs | - | [1] |
| rRNAs | Ribosomal RNAs | - | [3] |
Mass spectrometry analyses have revealed that glycoRNAs primarily contain fucosylated and sialylated complex glycans [2]. The glycosylation significantly alters the physical properties of the RNA, resulting in slower migration on gels due to the added mass and structural complexity of the glycans [2].
The Discovery of acp3U: The Glycan Anchoring Site
A critical breakthrough in understanding glycoRNA biochemistry came with the identification of the specific nucleotide modification that serves as the glycan attachment site. Researchers using RNA-specific periodate oxidation and aldehyde labeling (rPAL) combined with high-sensitivity mass spectrometry identified 3-(3-amino-3-carboxypropyl)uridine (acp3U) as a key nucleotide anchoring site for N-glycan linkage [1].
acp3U is a highly conserved modified uridine present in bacterial and mammalian tRNAs, previously known to enhance tRNA thermostability and play significant roles in cellular physiology [1]. Enzymes such as DTW domain-containing 2 (DTWD2) are essential for acp3U formation, and their absence significantly alters glycoRNA biosynthesis, reducing glycoRNA display on cell surfaces [3].
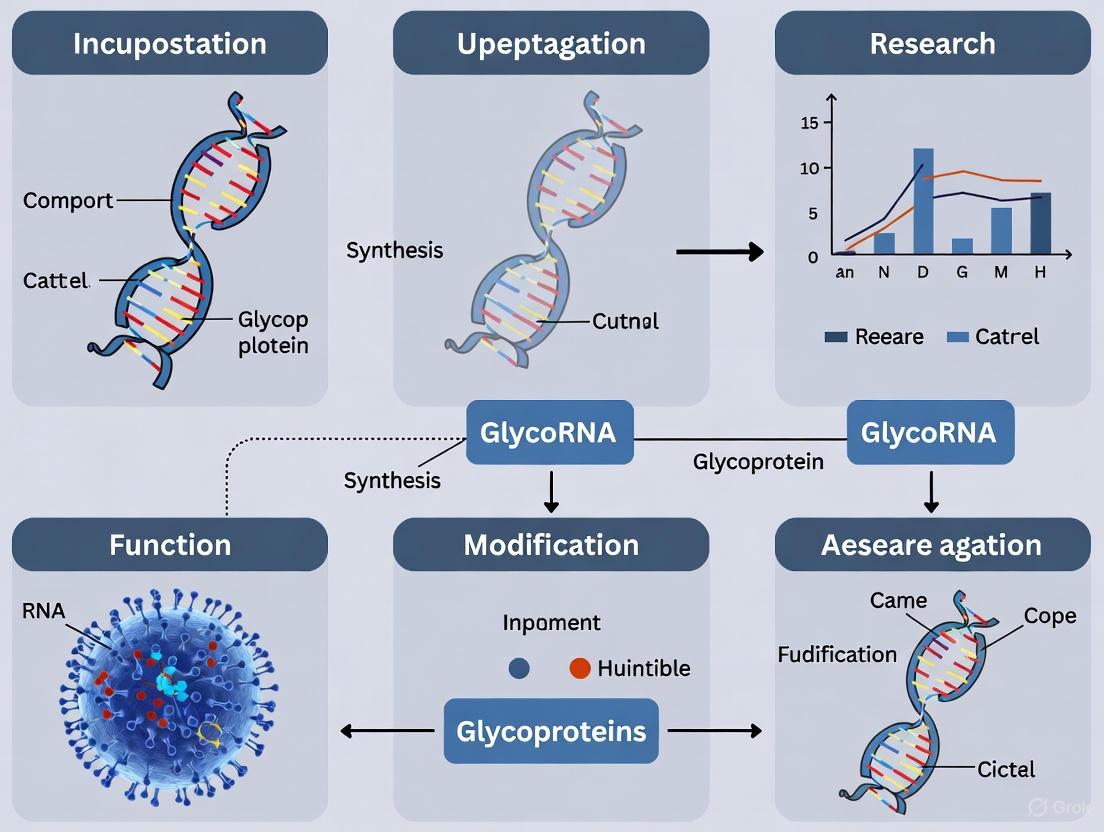
Figure 1: GlycoRNA Biosynthesis and Function Pathway. Key enzymes (red) catalyze specific steps in glycoRNA formation, leading to functional cell surface localization and immune interactions (green).
Methodological Advances in GlycoRNA Research
Detection and Visualization Techniques
The investigation of glycoRNAs has required the development of specialized detection methodologies that can simultaneously recognize RNA sequences and glycan structures.
Table 2: Key Methodologies for GlycoRNA Detection and Analysis
| Method | Full Name | Principle | Key Findings Enabled | References |
|---|---|---|---|---|
| rPAL | RNA-optimized periodate oxidation and aldehyde ligation | Leverages 1,2-diol reactivity in sialic acids for specific labeling | Identified acp3U as glycan attachment site | [1] |
| drFRET | Dual-recognition fluorescence resonance energy transfer | Visualizes glycosylated RNAs in small extracellular vesicles | Elucidated interactions with Siglec-10, Siglec-11, P-selectin | [1] |
| ARPLA | Aptamer and RNA in situ hybridization-mediated proximity ligation assay | Dual recognition of glycans and RNA triggers in situ ligation | Discovered intracellular trafficking via SNARE protein-mediated exocytosis | [1] |
| Ac4ManNAz Labeling | Metabolic labeling with N-azidoacetylmannosamine-tetraacylated | Azide-containing mannose derivative incorporates into glycans | Confirmed glycoRNA presence in glioma cells; enabled purification | [2] |
| Sequence-Specific RNA-Capture | Magnetic bead system with complementary probes | Enriches specific glycoRNAs (U2, U4, Y5) for analysis | Identified predominant glycoRNAs in glioma cells | [2] |
Experimental Workflow for GlycoRNA Isolation and Characterization
The following diagram outlines a standardized workflow for glycoRNA investigation, synthesized from multiple experimental approaches:
Figure 2: Experimental Workflow for GlycoRNA Analysis. This integrated approach combines metabolic labeling, biochemical enrichment, and functional validation to comprehensively study glycoRNAs.
Critical validation steps include enzyme sensitivity assays, where glycoRNA signals significantly decrease after treatment with sialidase, PNGase F, endo F2, and endo F3, confirming the glycoprotein-like nature of the modifications [2]. Additionally, RNase digestion experiments confirm the RNA composition of these molecules, as signals disappear upon RNase treatment but persist after DNase digestion [2].
Biosynthetic Pathways: Navigating a Cellular Paradox
The biosynthesis of glycoRNAs presents a fascinating biological paradox: while their glycan structures are synthesized via the endoplasmic reticulum-Golgi pathway—a process dependent on the oligosaccharyltransferase (OST) complex [1]—RNA molecules are not typically localized within these organelles. Several hypotheses have been proposed to resolve this paradox:
- RNA-Chaperone Hypothesis: Certain RNA-binding proteins (RBPs) may chaperone RNAs into or near the ER/Golgi compartments, facilitating access to enzymatic glycosylation machinery [3].
- Atypical Trafficking Routes: Unconventional vesicular transport or RNA-RBP complexes may allow RNA to transiently interact with ER/Golgi-associated glycosylation enzymes [3].
- Enzyme Relocation: Glycosylation machinery components might relocate to compartments accessible to RNA molecules under specific conditions.
Notably, the biosynthetic pathway for glycoRNAs appears distinct from O-GlcNAc modification catalyzed by O-GlcNAc transferase (OGT) and instead shares key enzymes with protein N-glycosylation pathways, including N-acetylgalactosaminyltransferases (GALNTs) and sialyltransferases [1] [3].
Functional Significance: From Immune Regulation to Cancer Biology
Role in Immune Recognition
GlycoRNAs have been confirmed to exist on the cell surface, where they interact with specific immune receptors [1]. Particularly significant is their interaction with sialic acid-binding immunoglobulin-like lectins (Siglecs), a family of immunoregulatory receptors [1] [3]. These interactions may facilitate immune checkpoint regulation and tumor immune evasion [1]. Additionally, glycoRNAs enhance neutrophil recruitment to inflammatory sites by interacting with P-selectin on endothelial cells, with their expression and function depending on the Sidt gene [1].
An emerging feature of glycoRNA biology involves their coordination with cell surface RNA-binding proteins (csRBPs). These csRBPs—including nucleolin, enolase, La protein, and others—form well-defined nanoclusters enriched with multiple RBPs and glycoRNAs [1]. These clustered structures can be disassembled by extracellular RNase treatment, indicating their dependence on RNA components [1]. The spatial organization of these glycoRNA-csRBP complexes may provide the structural foundation for precise immune recognition and responses.
Implications in Cancer Biology and Therapeutics
Table 3: GlycoRNA Alterations in Cancer Pathobiology
| Cancer Type | GlycoRNA Findings | Functional Consequences | References |
|---|---|---|---|
| Glioma | Abundant glycoRNAs, predominantly U2 and U4 snRNAs | Depletion inhibits glioma cell viability and proliferation | [2] |
| Breast Cancer | Surface glycoRNA levels inversely associated with malignancy | Non-tumorigenic cells show higher abundance than malignant cells | [3] |
| Multiple Cancers | Altered expression of glycoRNA-related enzymes (GALNT14, ST6GAL1) | Associated with poor prognosis and tumor progression | [3] |
GlycoRNAs contribute to multiple aspects of tumor development and progression. In glioma cells, depletion of cell-surface glycoRNAs significantly inhibits cell viability and proliferation without altering cell adhesion or apoptosis levels [2]. The progressive decrease in glycoRNA expression from non-tumorigenic to malignant and metastatic breast cancer cells suggests that reduced glycoRNA expression may be linked to increased tumor aggressiveness [3].
Enzymes involved in glycoRNA synthesis, such as GALNTs and sialyltransferases, are often aberrantly regulated in tumors and associated with poor prognosis [3]. For example, GALNT14, which influences O-glycosylation patterns, and ST6GAL1, an enzyme that adds sialic acid residues for N-glycosylation, are dysregulated in various cancers [3]. These enzymes represent promising candidates for further investigation of their impact on glycoRNA regulation in tumorigenesis.
The Scientist's Toolkit: Essential Research Reagents
Table 4: Essential Research Reagents for GlycoRNA Investigation
| Reagent/Category | Specific Examples | Function in GlycoRNA Research |
|---|---|---|
| Metabolic Labeling Agents | Ac4ManNAz (peracetylated N-azidoacetylmannosamine) | Incorporates azide groups into glycans for subsequent bioorthogonal conjugation and detection |
| Bioorthogonal Chemistry Reagents | DBCO-biotin, Streptavidin magnetic beads | Enables specific conjugation, pull-down, and purification of labeled glycoRNAs |
| Glycan-Degrading Enzymes | Sialidase, PNGase F, Endo F2, Endo F3 | Validates glycan-dependent signals through sensitivity experiments |
| Nucleases | RNase Cocktail, DNase I, RNase Inhibitors | Confirms RNA composition of signals and checks for DNA contamination |
| Sequence-Specific Capture Probes | U2, U4, Y5 antisense oligonucleotides | Enriches specific glycoRNA species for individual analysis |
| Analytical Tools | LC-MS/MS, Northern blot reagents, RNA-seq kits | Characterizes RNA and glycan composition of purified samples |
| Immunodetection Reagents | Anti-biotin streptavidin-HRP | Visualizes and detects biotin-labeled glycoRNAs after separation |
| Poricoic acid A | Poricoic Acid A | |
| Salvicine | Salvicine, CAS:240423-23-8, MF:C20H26O4, MW:330.4 g/mol | Chemical Reagent |
Future Perspectives and Therapeutic Horizons
The elucidation of glycoRNA function presents several promising avenues for therapeutic development:
Enzyme-Targeted Approaches: Targeting enzymes involved in glycoRNA biosynthesis, such as GALNTs and sialyltransferases, could manipulate glycoRNA production, potentially restoring immune recognition and inhibiting tumor growth [3].
Interaction Blockade: Developing monoclonal antibodies or small-molecule inhibitors to prevent glycoRNAs from interacting with immune inhibitory receptors like Siglecs could enhance anti-tumor immune responses [3].
Diagnostic Applications: GlycoRNAs may serve as novel biomarkers for cancer diagnosis and prognosis. Their unique presence in cancer cells and involvement in tumor-specific pathways make them attractive targets for diagnostic assays and imaging techniques [3].
Combination Therapies: Integrating glycoRNA-targeted approaches with existing immunotherapies, such as immune checkpoint inhibitors, may produce synergistic effects and improve patient outcomes [3].
However, significant challenges remain, including ensuring therapeutic specificity to avoid disrupting essential glycosylation pathways in healthy cells. Advanced delivery systems, such as nanoparticle-based vehicles and ligand-specific targeting strategies, will be crucial for focusing therapeutic agents on malignant cells while sparing normal tissues [3].
The discovery of glycoRNAs represents a genuine paradigm shift in molecular biology, fundamentally expanding our understanding of the functional repertoire of RNA and the complexity of cellular glycosylation. These novel biomolecules, residing at the interface of RNA biology and glycobiology, have emerged as significant players in immune regulation and cancer pathogenesis. While substantial progress has been made in characterizing their composition, biosynthesis, and functions, the field remains in its infancy. Ongoing research efforts to decipher the precise molecular mechanisms governing glycoRNA biogenesis and their multifaceted roles in physiology and disease will undoubtedly uncover new biological principles and create unprecedented opportunities for therapeutic intervention. The continued exploration of this emerging frontier promises to reshape our fundamental understanding of cellular communication and disease pathogenesis.
GlycoRNA represents a paradigm shift in cellular biology, establishing ribonucleic acid (RNA) as a third fundamental scaffold for glycosylation, alongside the long-recognized substrates of proteins and lipids [1] [4] [5]. This novel class of biomolecule consists of small non-coding RNAs (sncRNAs) covalently modified with sialylated and fucosylated N-glycans [1] [4]. Discovered in 2021, glycoRNAs are predominantly localized on the cell surface, challenging the traditional compartmentalization of nucleic acids within the cell and positioning them as potential key players in extracellular communication and immune recognition [4] [6] [5]. This technical guide deconstructs the core characteristics of glycoRNA, detailing its structural components, detection methodologies, and biological functions, thereby providing a foundational resource for research and therapeutic development.
Structural Composition: The Core of GlycoRNA
The RNA Backbone
The RNA component of glycoRNAs is primarily derived from conserved small non-coding RNAs. Sequencing of affinity-purified glycoRNAs has identified specific sncRNA families that serve as the backbone [1] [4].
Table 1: Primary Small Non-Coding RNA Species Identified in GlycoRNA Preparations
| sncRNA Type | Key Features | Representative Examples |
|---|---|---|
| Y RNA | Most abundantly glycosylated species; involved in RNA quality control and DNA replication [6]. | RNY1, RNY3, RNY4, RNY5 |
| Transfer RNA (tRNA) | Highly conserved; key role in protein translation [7]. | |
| Small Nuclear RNA (snRNA) | Involved in pre-mRNA splicing [1] [7]. | U1, U2, U4, U5, U6 |
| Small Nucleolar RNA (snoRNA) | Guides chemical modifications of other RNAs [1] [7]. | |
| MicroRNA (miRNA) | Key regulators of gene expression at the post-transcriptional level [1]. |
The Glycan Motif
The glycan component attached to these RNAs resembles canonical N-glycans typically found on glycoproteins [4] [8]. Key characteristics include:
- Composition: The structures are enriched in sialic acid and fucose at their termini, which are critical for receptor binding [1] [4].
- Biosynthesis: Assembly of the glycoRNA glycan depends on the canonical N-glycan biosynthetic machinery, including the oligosaccharyltransferase (OST) complex within the endoplasmic reticulum-Golgi pathway [1] [6].
- Tissue-Specific Profiles: GlycoRNA glycan structures exhibit tissue-specific variations, such as high-mannose forms in the heart and fucosylated forms in the brain [8].
The Covalent Linkage
The covalent linkage connecting the RNA and glycan was a central mystery initially met with skepticism [5]. Recent research has identified the modified nucleoside 3-(3-amino-3-carboxypropyl)uridine (acp3U) as the key anchoring site for N-glycan attachment on RNA [1]. acp3U is a highly conserved modified uridine present in bacterial and mammalian tRNAs, known to enhance RNA thermostability [1]. The discovery of this linker provides conclusive evidence for glycoRNA as a bona fide covalent glycoconjugate [5].
Methodological Toolkit: Detection and Analysis of GlycoRNAs
The study of glycoRNAs requires specialized techniques for their detection, visualization, and functional analysis. The following table summarizes key experimental reagents and their applications.
Table 2: Essential Research Reagents and Methods for GlycoRNA Analysis
| Reagent / Method | Core Function | Key Application in GlycoRNA Research |
|---|---|---|
| Metabolic Labeling (Acâ‚„ManNAz) | Incorporates azide-modified sialic acid into nascent glycans [9]. | Enables click chemistry-based tagging and pull-down of glycoRNAs [9] [4]. |
| rPAL (RNA-optimized Periodate oxidation and Aldehyde Ligatio) | Oxidizes 1,2-diols in sialic acids to generate aldehydes for covalent capture [1]. | Sensitive enrichment and isolation of native glycoRNAs; helped identify acp3U linkage [1]. |
| ARPLA (Aptamer and RNA in situ hybridization-mediated Proximity Ligation Assay) | Dual-recognition of glycans and RNA to trigger in situ amplification [1]. | High-sensitivity visualization of glycoRNAs at the single-cell level [1] [8]. |
| drFRET (dual-recognition FRET) | FRET-based imaging using dual recognition of glycosylated RNAs [1]. | Visualizing glycoRNAs on small extracellular vesicles (sEVs) from clinical samples [1]. |
| Mass Spectrometry (Coupled with rPAL) | High-sensitivity analysis of RNA modifications [5]. | Direct identification of the glycan-RNA linkage (e.g., acp3U) [1] [5]. |
| N4-Acetylcytidine | N4-Acetylcytidine, CAS:3768-18-1, MF:C11H15N3O6, MW:285.25 g/mol | Chemical Reagent |
| Urolithin B | Urolithin B, CAS:1139-83-9, MF:C13H8O3, MW:212.20 g/mol | Chemical Reagent |
Critical Experimental Workflow and Considerations
A generalized workflow for glycoRNA isolation and validation involves metabolic labeling, RNA extraction, click-chemistry conjugation to a reporter (e.g., biotin), affinity purification, and downstream analysis via sequencing or blotting [9] [4]. A critical methodological consideration is the potential for co-purifying glycoproteins, such as LAMP1, which can be a source of contaminating glycans in RNA preparations [9]. Robust protocols must include stringent controls, including:
- Proteinase K digestion under denaturing conditions to eliminate glycoprotein contaminants [9].
- RNase sensitivity assays to confirm the RNA-dependent nature of the signal [9] [4].
The diagram below illustrates a core experimental workflow for glycoRNA validation, highlighting key steps and necessary controls to ensure specificity.
Biological Function and Therapeutic Implications
Cell Surface Localization and Interaction with Immune Receptors
A defining feature of glycoRNAs is their presence on the outer leaflet of the plasma membrane [4] [5]. Their surface expression is dynamic, changing with cell state, such as decreasing as monocytes differentiate into macrophages but increasing upon LPS-induced activation [8]. On the cell surface, glycoRNAs function as ligands for specific immune receptors, primarily the Siglec (Sialic acid-binding immunoglobulin-type lectin) family [1] [4] [6]. Studies show that Siglec-11 and Siglec-14 exhibit binding that is vulnerable to RNase treatment, directly implicating glycoRNAs as ligands [4] [6]. This interaction positions glycoRNAs as potential novel players in immune checkpoint regulation and tumor immune evasion [1].
Role in Disease and Drug Targeting Potential
The dysregulation of glycoRNA expression is increasingly implicated in human diseases, making them emerging drug targets [8].
- Cancer: In acute myeloid leukemia (AML), glycoRNAs form nanoclusters on the cell surface with RNA-binding proteins like hnRNPU. These clusters promote interactions with Siglec receptors, contributing to immune evasion. Removing these surface glycoRNAs has been shown to enhance the phagocytosis of AML cells by macrophages, suggesting a promising therapeutic avenue [8].
- Inflammatory Diseases: GlycoRNAs mediate immune cell trafficking. For instance, they are essential for neutrophil recruitment to inflammatory sites and for the adhesion of monocytes/macrophages to endothelial cells, a critical step in inflammation [1] [8].
- Autoimmunity: Given that many RNA-binding autoantibodies are hallmarks of autoimmune diseases like lupus, and that glycoRNAs bind both Siglecs and anti-dsRNA antibodies, they are hypothesized to be a source of novel autoantigens, potentially illuminating new mechanisms in autoimmunity [6] [5].
The following diagram summarizes the documented and hypothesized roles of glycoRNA in the immune system.
GlycoRNA, a covalent conjugate of sialylated N-glycans and small non-coding RNA backbones, is a validated third class of glycoconjugate that demands a re-evaluation of long-standing biological principles [5]. Its presence on the cell surface and interactions with critical immunoregulatory receptors like Siglecs outline a direct interface between RNA biology and glycobiology [4] [10]. While fundamental questions regarding its precise biosynthetic pathway and full structural diversity remain active areas of research [9] [8], the accumulating evidence firmly establishes its role in cell communication and disease pathogenesis. For researchers and drug development professionals, glycoRNA represents not only a new frontier in basic science but also a promising and largely untapped landscape for diagnostic and therapeutic innovation in immunology and oncology.
The conventional secretory pathway, comprising the endoplasmic reticulum (ER) and Golgi apparatus, has long been established as the primary route for the processing and surface display of macromolecules. However, the recent discovery of glycoRNAs—small non-coding RNAs modified with glycans and present on the cell surface—presents a fundamental paradox. These molecules challenge the traditional dogma that RNA is confined intracellularly and necessitate a re-examination of the mechanisms through which diverse macromolecules traverse the secretory pathway to reach their final destinations. This whitepaper synthesizes current research to resolve this paradox, detailing the dynamic, cargo-adapted mechanisms of the ER-Golgi pathway, presenting definitive evidence for glycoRNAs, and exploring their implications for immune regulation and therapeutic development.
For decades, the secretory pathway has been understood through George Palade's seminal model: newly synthesized proteins in the ER are transported via vesicular intermediates to the Golgi apparatus for modification before reaching their final destinations [11]. This framework comfortably explained the surface localization of glycosylated proteins and lipids. The recent proposition of a third class of glycoconjugate—glycosylated RNAs (glycoRNAs)—fundamentally disrupts this paradigm [5]. The paradox is twofold: first, how RNA, traditionally considered an intracellular molecule, is glycosylated and displayed on the cell surface; and second, what specialized mechanisms within the ER-Golgi pathway facilitate this novel form of secretion. This guide delves into the sophisticated, cargo-specific adaptations of the secretory machinery that resolve this apparent contradiction, positioning glycoRNA research within a broader thesis of cellular trafficking and communication.
Dynamic Export Pathways at the Endoplasmic Reticulum
The ER serves as the entry point for the secretory pathway, but it is not a monolithic organelle. Recent research reveals specialized export mechanisms that diverge significantly from the classical model of uniform COPII vesicles.
Cargo-Dependent Export Mechanisms
The ER employs at least two distinct export routes, determined by the physical characteristics of the cargo.
- COPII Vesicles for Standard Cargo: Conventional COPII vesicles, approximately 60-80 nm in diameter, transport the majority of small, soluble secretory proteins from the ER to the Golgi complex [11] [12]. The assembly of these vesicles is a hierarchical process initiated by the activation of the small GTPase Sar1, which recruits the inner coat complex Sec23-Sec24, followed by the outer coat complex Sec13-Sec31 [12].
- Specialized Tunnels for Bulky Cargo: Large macromolecular assemblies like procollagen (∼300 nm), mucins, and ApoB-containing lipid particles are physically too large to fit into standard COPII vesicles [11]. These bulky cargoes are exported via a distinct mechanism involving TANGO1 (Transport ANd Golgi Organization 1). TANGO1 assembles into a ring-like structure at ER exit sites (ERES), where it collaborates with COPII components to form tubular tunnels or conduits that allow for the direct export of oversized cargo [11]. This process may also involve the formation of a transient tunnel between the ER and the ER-Golgi Intermediate Compartment (ERGIC) [11].
Table 1: Key Protein Machinery in ER Export Pathways
| Protein Complex | Primary Function | Key Cargo | Structural Features |
|---|---|---|---|
| COPII (Sec23/Sec24, Sec13/Sec31) | Forms small transport vesicles; induces membrane curvature [12]. | Small secretory proteins (e.g., ss-GFP) [11]. | Cuboctahedral cage structure; 60-80 nm diameter [11] [12]. |
| TANGO1/cTAGE5 | Organizes ring-like structures at ERES; nucleates formation of export tunnels for large cargo [11]. | Collagens, ApoB-lipid particles, mucins [11]. | Transmembrane protein with cytoplasmic coiled-coil domains and luminal SH3-like domain. |
| NRZ Complex | Tethers TANGO1 to ERGIC membranes, facilitating tunnel formation [11]. | Supports bulky cargo export. | Tethering complex linking ER and ERGIC. |
Evidence for Distinct ER Exit Sites
Quantitative studies demonstrate the existence of molecularly distinct ERES. Optical trapping of TANGO1 and Sec23 showed that while small cargo molecules are arrested at nearly 80% of ERES, bulky collagens are retained at only about 40% of these sites [11]. This finding confirms that the export of collagens is restricted to a specialized subset of exit sites, underscoring the compartmentalization of export pathways based on cargo size and identity.
Intra-Golgi Transport: Cisternal Maturation vs. Stable Compartments
Upon exiting the ER, cargo enters the Golgi apparatus, another hub of dynamic processing. The mechanism of intra-Golgi transport remains a subject of active investigation, with two primary models under consideration.
Competing Models of Golgi Function
- Cisternal Progression/Maturation Model: This model posits that Golgi cisternae themselves mature, moving from the cis to the trans position while carrying their cargo. Resident Golgi enzymes are recycled backwards via COPI vesicles [13].
- Stable Compartment Model: This model suggests that Golgi cisternae are stable entities, and cargo is actively transported between them via vesicular carriers or through dynamic rim domains, while the enzymes remain in place [13].
Quantitative Data Supporting the Stable Compartment Model
Recent quantitative imaging in nocodazole-induced Golgi ministacks provides compelling evidence for the stable compartment model. Key findings include:
- Cargo-Specific Transport Velocities: The intra-Golgi transport velocity of a secretory cargo decreases as it moves from the cis to the trans side, and different cargos exhibit distinct velocities even within the same cisternae [13].
- Vastly Different Golgi Residence Times: Different cargos have highly variable residence times within the Golgi. Remarkably, truncating the luminal domain of the transmembrane protein Tac extended its Golgi residence time from 16 minutes to 3.4 hours, indicating that cargo identity and structure directly influence trafficking kinetics [13].
- COPI-Independent Golgi Stability: When COPI-mediated retrograde transport was inhibited, Golgi ministacks remained intact for 30-60 minutes, challenging the notion that continuous retrograde vesicle flow is essential for maintaining Golgi structure [13].
These data suggest that the Golgi operates less like a conveyor belt and more like a series of stable processing stations, with cargo movement subject to active sorting and regulation.
The GlycoRNA Paradox: Existence, Evidence, and Secretory Route
The discovery of glycoRNA introduces a new molecule into the established framework of the secretory pathway, creating a compelling paradox.
Conclusive Evidence for GlycoRNA
Initial reports of glycoRNA in 2021 were met with skepticism, as the findings could potentially be explained by contaminating glycoproteins [9] [5]. However, recent research has provided definitive proof:
- Advanced Mass Spectrometry: The development of a highly sensitive mass spectrometry method identified multiple direct chemical linkers between an RNA base (specifically, the non-canonical base acp3U) and a sugar molecule, providing conclusive evidence of a covalent RNA-glycan conjugate [5].
- Elimination of Protein Contamination: While one study showed that glycoproteins like LAMP1 can co-purify with small RNA preparations using certain protocols [9], the application of proteinase K under denaturing conditions and the direct chemical evidence confirm that glycoRNAs exist as a distinct class of biomolecule [5].
Table 2: Experimental Evidence for and against GlycoRNA
| Study/Source | Key Findings | Interpretation |
|---|---|---|
| Flynn et al. 2021 (Initial Discovery) | Metabolic labeling with Ac4ManNAz detected glycans in small RNA preparations; signals sensitive to RNase but not proteinase K [9]. | Proposed existence of glycoRNA, but potential for protein contamination remained. |
| Opposing Study (2025) | Glycan signals in RNA preps were resistant to RNase but sensitive to proteinase K under denaturing conditions; identified co-purifying glycoproteins (e.g., LAMP1) [9]. | Argued that glycoproteins are a major source of glycan signal in "glycoRNA" samples. |
| Flynn et al. 2024 (Definitive Proof) | Used native chemical labeling and novel mass spectrometry to identify covalent linkages between acp3U RNA base and glycans [5]. | Provided direct, conclusive evidence for the existence of true glycoRNA molecules. |
Proposed Biogenesis and Trafficking of GlycoRNA
The pathway for glycoRNA biogenesis and surface localization is still being mapped, but it intersects with the canonical secretory pathway.
- Glycosylation Machinery: Evidence suggests that the N-glycosylation machinery, including the oligosaccharyltransferase (OST) complex, traditionally associated with protein glycosylation, is involved in glycoRNA modification [10] [14].
- Secretory Pathway Dependence: Given their glycosylation and cell surface localization, it is hypothesized that glycoRNAs transit through the ER and Golgi compartments. This would place them within the same dynamic, cargo-adapted pathways previously described for proteins and lipids, potentially utilizing specialized export mechanisms similar to those used by other bulky or atypical cargoes [11] [5].
- Immune Function: A leading hypothesis for glycoRNA function is immune regulation. Cell surface glycoRNAs have been shown to interact with sialic acid-binding immunoglobulin-type lectins (Siglecs), suggesting a role in self/non-self recognition that may be relevant in autoimmune diseases and cancer [14] [5].
Quantitative Imaging of Intracellular Transport
Understanding the localization paradox requires precise measurement of how molecules move through the cell. Recent advances in quantitative imaging have provided unprecedented insights into transport kinetics.
Methodology for Tracking Lipid Transport
A groundbreaking 2025 study established a pipeline to map species-specific lipid transport and metabolism [15]:
- Probe Design and Loading: Bifunctional, photoactivatable lipid probes (PC, PE, PA, SM) with minimal modifications were loaded into the plasma membrane (PM) of U2OS cells via α-methyl-cyclodextrin-mediated exchange from donor liposomes [15].
- Pulse-Chase and Crosslinking: After a chase period (0 min to 24 h), lipids were photo-crosslinked in situ, cells were fixed, and non-crosslinked lipids were removed.
- Visualization and Quantification: Crosslinked lipids were labeled via click chemistry for confocal imaging. The fluorescence signal was assigned to specific organelles (PM, Golgi, ER, endosomes, mitochondria) using segmented probability maps generated with the Ilastik software package [15].
- Metabolic Tracking: Parallel samples were analyzed by ultra-high-resolution Fourier-transform mass spectrometry (FT MS) to track the metabolic conversion of the lipid probes over time [15].
Key Findings on Lipid Sorting
This approach yielded the first quantitative map of retrograde lipid flux, revealing that:
- Non-vesicular Transport Dominates Sorting: Directional, non-vesicular lipid transport was found to be responsible for fast, species-selective lipid sorting. This process was up to 11 times faster than slow, non-specific vesicular trafficking [15].
- Species-Specific Kinetics: Poly-unsaturated PC species and PE exhibited rapid relocation to the ER, while saturated PC species and sphingomyelin were retained longer in the PM and endosomes [15].
- Coupling to Energetics: The study identified a coupling between energy-dependent lipid flipping and non-vesicular transport as a core mechanism for directional lipid sorting [15].
Table 3: Retrograde Transport Kinetics of Selected Lipid Species [15]
| Lipid Species | Primary Transport Route | Relative Transport Rate (Non-vesicular) | Key Organelle Destinations |
|---|---|---|---|
| PE(18:1/Y) | Non-vesicular (PM→ER) | Fastest | ER |
| PC(18:1/Y-18:1) | Non-vesicular (PM→ER) | Fast | ER, Golgi |
| SM(d18:1/Y) | Non-vesicular (PM→ER) | Moderate | PM, Endosomes |
| PC(16:0/Y) | Non-vesicular (PM→ER) | Slowest | PM, Endosomes |
The Scientist's Toolkit: Key Reagents and Protocols
This section details essential reagents and methods for investigating the secretory pathway and glycoRNA.
Research Reagent Solutions
Table 4: Essential Reagents for Secretory Pathway and GlycoRNA Research
| Reagent / Tool | Function / Application | Key Characteristics / Targets |
|---|---|---|
| Bifunctional Lipid Probes | Quantitative imaging of lipid transport and metabolism [15]. | Minimal diazirine and alkyne modifications; resemble native lipids (e.g., PC, PE, PA, SM). |
| Metabolic Label (Ac4ManNAz) | Incorporates azido-sialic acid into nascent N-glycans for click chemistry-based detection [9]. | Key for labeling glycoRNA and other glycoconjugates. |
| TANGO1 Depletion (siRNA/shRNA) | Functional studies of bulky cargo export from the ER [11]. | Inhibits secretion of collagens, ApoB particles; causes ER retention. |
| COPII Inhibitors (Sec31 mutants) | Probing conventional ER-to-Golgi vesicular transport [12]. | e.g., Trp922Ala/Asn923Ala mutations impair Sec23 GAP-enhancement. |
| Nocodazole | Induces Golgi ministacks for quantitative imaging of intra-Golgi transport [13]. | Disrupts microtubules, leading to uniform, rotationally symmetrical Golgi fragments. |
| Ilastik Software | Pixel classification and organelle segmentation for quantitative image analysis [15]. | Generates probability maps to assign fluorescence signals to specific organelles. |
| trans-3-(3-Pyridyl)acrylic acid | trans-3-(3-Pyridyl)acrylic acid, CAS:19337-97-4, MF:C8H7NO2, MW:149.15 g/mol | Chemical Reagent |
| L-Fuco-4-O-methyl-D-glucurono-D-xylan | L-Fuco-4-O-methyl-D-glucurono-D-xylan | L-Fuco-4-O-methyl-D-glucurono-D-xylan is a complex acidic xylan for plant polymer research. This product is For Research Use Only. Not for human use. |
Detailed Experimental Protocol: GlycoRNA Isolation and Validation
To address the controversy surrounding glycoRNA, a rigorous protocol is essential [9]:
- Metabolic Labeling: Incubate cells (e.g., HeLa, NIH3T3) with 100 µM Ac4ManNAz in culture medium for 40 hours.
- Total RNA Extraction: Lyse cells in TRIzol reagent. Perform phase separation with chloroform and precipitate RNA from the aqueous phase with isopropanol.
- Silica Column Purification: Desalt and purify the RNA pellet using Zymo Spin IICG or IIC columns with multiple wash steps (RNA Prep Buffer, 80% ethanol). Elute with ultrapure water.
- Robust Protein Digestion: Treat the purified RNA sample (e.g., 25 µg) with proteinase K (1 µg) under denaturing conditions (e.g., in Denaturing Tris Buffer with SDS and 2-mercaptoethanol) at 37°C for 45 minutes. This critical step eliminates contaminating glycoproteins.
- Validation via Click Chemistry: Perform strain-promoted azide-alkyne cycloaddition (SPAAC) to conjugate a biotin reporter to the metabolically labeled azido-glycans.
- Detection: Analyze via Northern blotting with streptavidin probes and/or confirm via advanced mass spectrometry to identify the covalent RNA-glycan linkage.
Visualizing the Pathways: Diagrams
The following diagrams illustrate the core concepts and experimental workflows discussed in this whitepaper.
Cargo-Specific ER Export Pathways
Quantitative Lipid Transport Imaging Workflow
GlycoRNA Validation and Controversy Resolution
The localization paradox of cell surface display is resolved by recognizing the profound adaptability of the ER-Golgi pathway. The secretory machinery is not a rigid conveyor belt but a dynamic, responsive system capable of deploying specialized mechanisms—from TANGO1-gated tunnels for collagen to non-vesicular transport for selective lipid sorting—to handle diverse molecular cargo. The confirmed existence of glycoRNA adds a fascinating new dimension to this system, implying yet another specialized export route.
Future research must focus on elucidating the precise molecular machinery responsible for glycoRNA glycosylation and trafficking. Furthermore, the functional implications of cell surface glycoRNAs, particularly their role as ligands for immune receptors like Siglecs, open a new frontier in immunology and therapeutic development [14] [5]. Understanding how these novel molecules are generated and trafficked through the classic secretory pathway will not only resolve the current paradox but also likely reveal new principles of cellular organization and communication.
The conceptual framework of cellular glycosylation has undergone a fundamental expansion. Traditionally, glycosylation—the enzymatic process of attaching glycans to biomolecules—was considered exclusive to proteins and lipids, playing critical roles in structural integrity, cellular recognition, and signaling. The seminal discovery that RNA can be glycosylated has introduced a third class of glycoconjugate: glycoRNA [1] [5]. This finding bridges the previously distinct fields of RNA biology and glycobiology, suggesting a more complex landscape of cellular communication than previously appreciated.
GlycoRNAs are primarily small non-coding RNAs—including Y RNAs, snoRNAs, and tRNAs—modified with sialylated and fucosylated N-glycans and displayed on the outer surface of cell membranes [1] [16]. Their extracellular localization positions them as potential mediators of intercellular communication, particularly with components of the immune system such as Siglec receptors [1]. The central mystery, however, remained the precise molecular linkage between the RNA molecule and the complex glycan structure. Recent research has identified 3-(3-amino-3-carboxypropyl)uridine (acp3U), a modified uridine, as a primary attachment site, providing a mechanistic foundation for this novel biological phenomenon [17] [18].
acp3U: The Chemical Linchpin of GlycoRNA Biogenesis
The Discovery and Chemical Nature of acp3U
The identification of acp3U as the glycan attachment site represents a convergence of advanced chemical biology and analytical techniques. acp3U is not a novel nucleoside; it was first described five decades ago as a conserved modified uridine found in bacterial and mammalian tRNAs [17] [18]. Its chemical structure features a unique 3-amino-3-carboxypropyl side chain extending from the uracil base, providing a functional handle not present in canonical nucleotides [17].
This side chain terminates in both amine and carboxylic acid functional groups, making it a plausible candidate for forming stable linkages with glycans [17]. Prior to its implication in glycoRNA, acp3U was known to contribute to tRNA thermostability and play significant roles in cellular physiology [1] [17]. The discovery that this well-characterized modification serves as an attachment point for complex glycans demonstrates how new biological functions can be discovered for known chemical entities.
Experimental Validation of acp3U as the Glycan Attachment Site
The assignment of acp3U as the primary glycan attachment site was established through a multi-faceted experimental approach, with key validation data summarized in the table below.
Table 1: Key Experimental Evidence Establishing acp3U as the Glycan Attachment Site
| Experimental Approach | Key Findings | Interpretation |
|---|---|---|
| rPAL with SWATH-MS [17] [18] | Identified acp3U as the most abundant carboxylate-containing nucleoside across enzymatic release approaches and cell lines. | acp3U is a predominant modified nucleoside in glycoRNA preparations. |
| Amidase Digestion in H₂¹â¸O [17] | Showed the expected mass increase for MS signals, confirming hydrolysis of an amide linkage. | Glycans are attached to RNA via an amide bond. |
| Endoglycosidase Treatment [17] | Revealed the presence of acp3U-GlcNAc. | Direct evidence of a glycan (GlcNAc) conjugated to acp3U. |
| DTWD2 Knockout Studies [18] | Resulted in decreased acp3U levels and reduced glycoRNA signal. | The enzyme responsible for installing acp3U is essential for glycoRNA biogenesis. |
| PNGase F Treatment [18] | Caused a substantial molecular weight shift of glycoRNA and released glycosylated acp3U. | Confirms acp3U is a direct target of N-glycosylation. |
The evidence collectively demonstrates that acp3U serves as a direct template for N-glycosylation, with its side chain carboxylic acid forming an amide bond with the glycan moiety [17] [18].
Methodological Advances: Detecting and Analyzing glycoRNA-acp3U Linkage
RNA-Optimized Periodate Oxidation and Aldehyde Labeling (rPAL)
The development of RNA-optimized periodate oxidation and aldehyde labeling (rPAL) represents a significant technical advancement that enabled the precise characterization of the glycoRNA-acp3U linkage [17] [18]. This method provides a more direct approach to labeling native sialoglycoRNAs compared to prior metabolic labeling strategies.
The rPAL workflow capitalizes on the distinct reactivity of 1,2-diols present in sialic acid residues of glycans [1] [17]. Periodate oxidation converts these vicinal diols into aldehyde groups, which subsequently form stable oxime ligation products with aminooxy-containing reagents or solid supports. This specific chemistry allows for efficient enrichment of sialylated glycoRNAs from complex biological samples [17].
When compared to the earlier metabolic labeling method using Ac4ManNAz, rPAL demonstrates remarkable improvements in sensitivity, achieving up to a 25-fold improvement in signal recovery per RNA mass and a 1,503-fold increase in signal sensitivity [18]. This enhanced sensitivity is crucial for detecting low-abundance glycoRNAs and for subsequent analysis of their composition and linkage.
Experimental Workflow for acp3U-GlycoRNA Analysis
The following diagram illustrates the integrated experimental workflow used to discover and validate acp3U as the key glycan attachment site in glycoRNA.
Critical Research Reagents and Tools
The investigation of acp3U and glycoRNA biology relies on a specialized set of research reagents and methodologies. The following table details essential components of the methodological toolkit.
Table 2: Essential Research Reagent Solutions for GlycoRNA-acp3U Investigation
| Reagent/Method | Specific Function | Key Utility |
|---|---|---|
| rPAL (RNA-optimized periodate oxidation and aldehyde labeling) [17] [18] | Selective enrichment of sialylated glycoRNAs via oxidation of sialic acid 1,2-diols and oxime ligation. | Direct labeling of native structures; 25-fold higher signal vs. metabolic labeling. |
| SWATH-MS Mass Spectrometry [17] | Data-independent acquisition LC-MS/MS for comprehensive nucleoside identification and quantification. | Sensitive detection and validation of modified nucleosides like acp3U. |
| DTWD2 Knockout Cell Lines [18] | Genetic disruption of enzyme installing acp3U into tRNA. | Validates essential role of acp3U in glycoRNA biogenesis. |
| Glycosidase Enzymes (PNGase F, Amidases) [17] [18] | Enzymatic cleavage of N-glycans or amide bonds between RNA and glycan. | Confirms chemical nature of RNA-glycan linkage. |
| GlycoRNAdb Database [16] | Curated repository of glycoRNA sequences, structures, abundance, and glycan information. | Resource for data exploration and hypothesis generation. |
Biological Implications: From Molecular Structure to Immune Function
Proposed Biogenesis Pathway for acp3U-Modified GlycoRNA
The discovery of acp3U as a glycan attachment site enables a more detailed model of glycoRNA biogenesis. While many details remain to be fully elucidated, current evidence suggests a pathway involving sequential enzymatic activities.
The process is proposed to initiate with the installation of acp3U into precursor RNAs by the enzyme DTWD2 [18]. This modification likely occurs within the nucleus or cytosol as part of standard RNA processing. The subsequent amidation of the acp3U side chain is hypothesized to create a suitable substrate for the oligosaccharyltransferase (OST) complex, which transfers the core N-glycan from a lipid-linked dolichol carrier [1] [17]. This crucial step directly links glycoRNA biogenesis to the canonical endoplasmic reticulum-Golgi N-glycosylation machinery previously thought dedicated to proteins.
Following initial glycosylation, the maturing glycoRNA traffics through the secretory pathway, where the N-glycan undergoes further processing and modification by glycosyltransferases (e.g., sialyltransferases) to achieve its final complex structure [17]. Finally, the mature glycoRNA is transported to and displayed on the cell surface via SNARE protein-mediated exocytosis, positioning it for potential interactions with extracellular binding partners [1].
Immune Regulatory Functions of acp3U-Modified GlycoRNA
The biological significance of acp3U glycosylation extends to a critical role in immune system regulation, particularly in distinguishing self from non-self. Recent research reveals that the unmodified acp3U base itself possesses intrinsic immunostimulatory properties capable of activating innate immune sensors [19]. The attachment of an N-glycan to acp3U effectively "cages" this immunostimulatory base, preventing it from triggering an inflammatory response against self-tissues [19].
This mechanism allows glycoRNAs to be displayed on the cell surface and within the endosomal network without inducing autoimmunity. This function is particularly relevant in the context of Siglec receptors (e.g., Siglec-10, Siglec-11), which are known to bind sialic acid residues on glycoRNAs and transmit inhibitory signals that dampen immune activation [1] [14]. The diagram below illustrates this immune regulatory mechanism.
This model provides a compelling biological rationale for why RNA undergoes glycosylation: to prevent inappropriate immune activation against self-RNA while potentially enabling specific immunoregulatory communication through receptor engagement [19]. Disruption of this system, through either reduced glycosylation or enhanced exposure of acp3U, could contribute to the development of autoimmune disorders such as lupus [19].
Current Challenges and Future Research Directions
Despite significant progress, the field of glycoRNA biology faces several challenges that warrant further investigation. Current detection methods, including rPAL, primarily target sialic acid-containing glycoRNAs, potentially missing glycoRNAs with different glycan compositions [17]. The periodate oxidation step can also non-specifically oxidize the 2',3' vicinal diols at the 3' terminus of RNA, though to a lesser extent [17]. Furthermore, distinct enzymatic release methods have yielded different relative abundances of modified nucleosides, suggesting a degree of protocol bias in current analyses [17].
Future research will need to address these technical limitations while exploring several key biological questions. These include elucidating the complete enzymatic pathway responsible for attaching glycans to acp3U, understanding the regulation of RNA glycosylation across different cellular conditions, and mapping the full glycoRNAome across diverse cell types and physiological states [18]. The development of new tools, such as single-cell spatial transcriptomics and advanced imaging approaches like ARPLA (Aptamer and RNA in situ hybridization-mediated Proximity Ligation Assay), will be crucial for these endeavors [1] [18].
From a therapeutic perspective, the established link between acp3U, glycoRNA, and immune regulation opens promising avenues for drug development, particularly for autoimmune diseases and cancer [1] [19]. As the fundamental mechanisms become clearer, researchers may identify specific enzymes in the glycoRNA synthesis pathway or receptor interactions that could be therapeutically targeted to modulate immune responses in pathological conditions.
Glycosylated RNA (glycoRNA), a recently discovered class of biomolecules, challenges traditional paradigms of cellular biology by demonstrating that RNA can be modified with complex glycans and localize to the cell surface. This whitepaper synthesizes current research on glycoRNA's primary functions, focusing on its newly defined roles in immune regulation and cell-cell communication. We detail the experimental methodologies enabling glycoRNA detection, analyze its interactions with key immune receptors, and explore its implications for cancer biology and therapeutic development. The emergence of glycoRNA as a third class of glycoconjugate alongside glycoproteins and glycolipids opens new avenues for understanding cellular communication and developing diagnostic and therapeutic strategies.
GlycoRNAs represent a novel category of biomolecules consisting of small non-coding RNAs covalently modified with N-glycans [3] [1]. This discovery has fundamentally expanded the scope of glycosylation beyond traditional substrates (proteins and lipids) to include nucleic acids [5]. These glycosylated molecules predominantly localize to the extracellular surface of the plasma membrane, positioning them uniquely to participate in extracellular recognition events [20].
The structural composition of glycoRNAs involves specific small non-coding RNA species—including Y RNAs, small nuclear RNAs (snRNAs), ribosomal RNAs (rRNAs), small nucleolar RNAs (snoRNAs), and transfer RNAs (tRNAs)—modified with sialylated and fucosylated N-glycan structures [3] [1]. Recent research has identified 3-(3-amino-3-carboxypropyl)uridine (acp3U), a modified uridine present in tRNAs, as a critical nucleotide anchoring site for N-glycan attachment [1]. The biosynthetic pathway of glycoRNAs appears to involve the endoplasmic reticulum-Golgi apparatus and utilizes elements of the canonical N-linked glycosylation machinery, including the oligosaccharyltransferase (OST) complex [1].
The surface localization and glycan composition of glycoRNAs enable their participation in essential biological processes, particularly immune cell recognition and intercellular signaling, which form the focus of this technical review.
GlycoRNA in Immune Recognition
Receptor Interactions and Signaling Mechanisms
GlycoRNAs function as ligands for several immunoregulatory receptors, most notably sialic acid-binding immunoglobulin-like lectins (Siglecs). These interactions play a crucial role in modulating immune responses:
Siglec Family Interactions: GlycoRNAs bind specifically to Siglec receptors, a family of immunoregulatory proteins expressed on various immune cells [1]. These interactions are mediated through the sialylated glycans present on glycoRNAs, similar to how glycoproteins engage Siglecs [3]. The binding between glycoRNAs and Siglecs can transmit inhibitory signals that dampen immune activation, potentially contributing to immune evasion mechanisms in pathological contexts [3].
Specific Siglec Partnerships: Research has identified interactions between glycoRNAs and specific Siglec family members, including Siglec-10 and Siglec-11 [1]. These receptors are known to function as immune checkpoints, suggesting that glycoRNAs may participate in the same regulatory pathways targeted by modern immunotherapies [1].
Autoimmune Implications: GlycoRNAs can bind to anti-double-stranded RNA antibodies, suggesting their potential involvement in autoimmune responses where anti-RNA antibodies are prevalent [3]. This interaction indicates that glycoRNAs may contribute to the loss of self-tolerance in autoimmune conditions by presenting RNA epitopes in an immunogenic context.
The following diagram illustrates the primary signaling pathways through which glycoRNAs mediate immune recognition:
Functional Immune Consequences
The receptor interactions described above translate into specific immunological outcomes with potential therapeutic relevance:
Neutrophil Recruitment: GlycoRNAs enhance neutrophil recruitment to inflammatory sites through their interaction with P-selectin on endothelial cells [1] [21]. This process depends on Sidt genes, which may facilitate the transport or surface display of glycoRNAs [1]. Neutrophil recruitment represents a well-characterized example of glycoRNA-mediated immune cell trafficking.
Tumor Immune Evasion: Cancer cells potentially exploit glycoRNA-Siglec interactions to suppress anti-tumor immunity [3]. By engaging inhibitory Siglec receptors on immune cells, tumor-derived glycoRNAs may create an immunosuppressive microenvironment that facilitates tumor progression and resistance to therapy.
Inflammatory Regulation: The presence of glycoRNAs on various cell types suggests their potential role in fine-tuning inflammatory responses across different tissues [3]. The tissue-specific glycan diversity observed in glycoRNAs may allow for specialized immunoregulatory functions in different organ systems.
GlycoRNA in Intercellular Communication
Mechanisms of Cell-Cell Signaling
GlycoRNAs participate in intercellular communication through multiple mechanisms:
Direct Cell Surface Interactions: As components of the cell glycocalyx, glycoRNAs engage in direct trans-cellular interactions with receptors on opposing cells [20]. This mode of signaling is particularly relevant for immune cell recognition and endothelial-leukocyte interactions.
Extracellular Vesicle-Mediated Signaling: GlycoRNAs are present on small extracellular vesicles (sEVs), where they facilitate vesicle targeting and cellular uptake [21]. The drFRET detection method has confirmed that sEV glycoRNAs specifically interact with Siglec proteins and P-selectin, which are critical for sEV cellular internalization [21]. This vesicular transport represents an important pathway for glycoRNA-mediated communication at a distance.
Cell Surface RNA-Binding Protein Complexes: GlycoRNAs form functional complexes with cell surface RNA-binding proteins (csRBPs) that assemble into defined nanoclusters on the extracellular surface [3] [1]. These clusters include proteins such as nucleolin, enolase, La protein, U5 SNRNP200, DDX21, hnRNPU, and NPM1, despite their lack of transmembrane domains [1]. The clustering of csRBPs with glycoRNAs enhances interactions with immunoregulatory receptors and provides spatial organization for precise immune recognition [3].
Biological Communication Contexts
The communication mechanisms described above enable glycoRNA participation in specific physiological and pathological processes:
Neutrophil-Endothelial Interactions: GlycoRNAs on neutrophils mediate interactions with endothelial cells, directly regulating neutrophil recruitment during inflammation [22]. This function has been demonstrated in vivo, establishing glycoRNAs as functional mediators of leukocyte trafficking.
Tumor Microenvironment Remodeling: Cancer cells may use glycoRNAs to communicate with stromal cells and remodel the tumor microenvironment, promoting tumor growth and therapy resistance [3]. Changes in glycoRNA expression can affect glycan-mediated signaling pathways that influence angiogenesis, immune cell recruitment, and stromal cell activation.
Extracellular Vesicle Targeting: The presence of specific glycoRNAs on sEVs determines their cellular tropism and biological activity [21]. This targeting function enables specialized communication between specific cell types within complex tissues.
Experimental Methodologies for GlycoRNA Detection
Metabolic Labeling and Northwestern Blot
The most established protocol for glycoRNA detection involves metabolic labeling followed by northwestern blot analysis [22]:
Metabolic Labeling: Cells are incubated with N-azidoacetylmannosamine-tetraacylated (Ac4ManNAz), a clickable sugar precursor that incorporates azide-modified sialic acid into nascent glycans [22]. Standard conditions use 100 μM Ac4ManNAz for 36-40 hours, though duration may require optimization for different cell types [22] [9].
RNA Extraction and Purification: High-purity RNA is prepared using TRIzol extraction, which removes proteins and hydrophobic contaminants while preserving small RNAs [22]. Subsequent purification steps include ethanol precipitation, desalting through FastPure RNA columns, and protein contamination removal via high-concentration proteinase K digestion [21]. Silica column purification (e.g., Zymo Research RNA Clean & Concentrator kits) is critical for removing unconjugated click chemistry reagents [22] [9].
Click Chemistry Labeling: Purified RNA is incubated with dibenzocyclooctyne-polyethylene-glycol-4-biotin (DBCO-PEG4-biotin) for copper-free click chemistry conjugation [22]. Standard reactions proceed at 25°C, followed by denaturation in formamide at 65°C [21].
Detection and Analysis: Labeled RNAs are separated by denaturing gel electrophoresis, transferred to nitrocellulose membranes, and detected via streptavidin-based blotting [22]. Optimization of blocking conditions (e.g., using EveryBlot or Intercept blocking buffers) and RNA staining (avoiding over-diluted SYBR Gold) is critical for signal specificity [22].
The following workflow diagram illustrates the key steps in this detection protocol:
Advanced Detection Technologies
Several innovative methods have expanded the glycoRNA detection toolkit:
drFRET (Dual-recognition FRET): This technique uses dual nucleic acid probes—one for glycan recognition (Neu5Ac probe) and another for RNA detection (in situ hybridization probe)—enabling sensitive, selective profiling of glycoRNAs on small extracellular vesicles from minimal biofluid samples (10 μL) [21]. drFRET achieves visualization of multiple glycoRNAs with high sensitivity and specificity through dipole-dipole coupling that prevents false-positive signals [21].
rPAL (RNA-optimized Periodate oxidation and Aldehyde Labeling): This method leverages periodate oxidation of 1,2-diols in sialic acids to generate aldehyde groups that form stable oxime bonds with aminooxy-functionalized solid-phase supports [1]. Combined with high-sensitivity mass spectrometry, rPAL identified acp3U as the key nucleotide anchoring site for glycan attachment [1].
ARPLA (Aptamer and RNA in situ hybridization-mediated Proximity Ligation Assay): ARPLA provides high-sensitivity visualization of glycoRNAs at the single-cell level through dual recognition of glycans and RNA to trigger an in situ ligation reaction [1]. This technique revealed that glycoRNAs undergo intracellular trafficking via SNARE protein-mediated secretory exocytosis [1].
Clier-qPCR (Click chemistry-based enrichment of glycoRNAs RT-qPCR): This method integrates click chemistry-based enrichment with real-time quantitative PCR to specifically validate and quantify glycoRNAs [23]. The approach captures biotin-labeled glycoRNAs with streptavidin magnetic beads followed by RT-qPCR analysis, enabling detection of low-abundance glycoRNAs (50-2000 nucleotides) with high specificity [23].
Research Reagent Solutions
Table 1: Essential Research Reagents for GlycoRNA Investigation
| Reagent Category | Specific Examples | Function and Application |
|---|---|---|
| Metabolic Labeling Reagents | Ac4ManNAz (N-azidoacetylmannosamine-tetraacylated) [22]; Ac4GalNAz (N-azidoacetylgalactosamine-tetraacylated) [21] | Incorporates clickable azide groups into cellular glycans for subsequent detection and purification |
| Click Chemistry Reagents | DBCO-PEG4-biotin (Dibenzocyclooctyne-PEG4-biotin conjugate) [22] | Enables copper-free click chemistry conjugation with azide-labeled glycans for biotin tagging |
| RNA Purification Kits | Zymo Research RNA Clean & Concentrator kits [22]; TRIzol reagent [22] [9] | Isolates high-purity RNA while removing contaminating proteins and lipids |
| Enzymatic Reagents | Proteinase K (under denaturing conditions) [9]; RNase A/T1 [22] | Confirms glycoconjugate identity through specific digestion patterns |
| Detection Reagents | High-sensitivity streptavidin-HRP [22]; SYBR Gold nucleic acid gel stain [22]; EveryBlot/Intercept blocking buffers [22] | Enables visualization and quantification of labeled glycoRNAs |
| Cell Culture Reagents | Cell-specific culture media; Recombinant murine IL-3 (for Ba/F3 cells) [22] | Maintains relevant cell models for glycoRNA studies |
GlycoRNA in Cancer Biology and Therapeutic Applications
Implications in Cancer Progression
GlycoRNAs contribute to multiple aspects of tumor development and progression:
Inverse Association with Tumor Aggressiveness: Surface glycoRNA levels are inversely associated with tumor malignancy and metastasis in cancer cell lines [3]. Non-tumorigenic breast cells exhibit higher glycoRNA abundance compared to malignant and metastatic breast cancer cells, which show progressively lower glycoRNA signals [3]. This suggests decreased glycoRNA expression may be linked to increased tumor aggressiveness.
Enzyme Expression Alterations: Enzymes involved in glycosylation, such as GALNTs and sialyltransferases (e.g., ST6GAL1), are aberrantly regulated in tumors and associated with poor prognosis [3]. These enzymes may influence glycoRNA synthesis or alter glycan composition, providing cancer cells with new mechanisms to regulate gene expression post-transcriptionally.
Tumor Microenvironment Interactions: GlycoRNAs may interact with stromal cells and contribute to remodeling of the tumor microenvironment, promoting tumor growth and resistance to therapy [3]. Changes in glycoRNA expression can affect glycan-mediated signaling pathways that influence angiogenesis, immune cell recruitment, and stromal cell activation.
Diagnostic and Therapeutic Potential
The unique properties of glycoRNAs present several promising clinical applications:
Cancer Diagnostics: GlycoRNA profiles on small extracellular vesicles achieve 100% accuracy in distinguishing cancers from non-cancer cases and 89% accuracy in classifying specific cancer types in a 100-patient cohort across six cancer types [21]. This diagnostic potential leverages the tissue-specific glycan diversity observed in glycoRNAs [20].
Therapeutic Targeting Strategies:
- Enzyme Inhibition: Targeting enzymes involved in glycoRNA biosynthesis, such as GALNTs and sialyltransferases, could manipulate glycoRNA production to restore immune recognition and inhibit tumor growth [3].
- Interaction Blockade: Developing monoclonal antibodies or small-molecule inhibitors to prevent glycoRNAs from interacting with immune inhibitory receptors could enhance anti-tumor immunity [3].
- Combination Therapies: Integrating glycoRNA-targeted approaches with existing immunotherapies, such as immune checkpoint inhibitors, may produce synergistic effects and improve patient outcomes [3].
Biomarker Development: GlycoRNAs show promise as novel biomarkers for cancer diagnosis and prognosis due to their unique presence in cancer cells and involvement in tumor-specific pathways [3]. Their detection could aid in early cancer detection, monitoring disease progression, and evaluating therapeutic responses.
Table 2: Quantitative Diagnostic Performance of sEV GlycoRNAs in Cancer Detection
| Diagnostic Application | Patient Cohort | Accuracy | Confidence Interval | Reference |
|---|---|---|---|---|
| Cancer vs. Non-cancer Discrimination | 100 patients (6 cancer types + controls) | 100% | 95% CI | [21] |
| Specific Cancer Type Classification | 100 patients (6 cancer types + controls) | 89% | Not specified | [21] |
| Cancer Aggressiveness Assessment | Breast cancer cell lines | Inverse correlation | Not specified | [3] |
Technical Considerations and Methodological Challenges
Research in the emerging field of glycoRNA biology presents several technical challenges that require careful consideration:
Contamination Control: Glycoproteins represent a considerable source of glycans that can copurify with RNA using current protocols [9]. Glycosylated membrane proteins such as LAMP1 can contaminate small RNA preparations and show resistance to RNase A/T1 treatment but sensitivity to proteinase K digestion under denaturing conditions [9]. Implementing rigorous controls including denaturing proteinase K treatments is essential for specificity.
RNA Integrity Preservation: The predominantly small RNA nature of glycoRNAs necessitates preservation of small RNA species during extraction [22]. TRIzol-based methods effectively preserve small RNAs while removing proteins and hydrophobic contaminants [22].
Detection Optimization: Appropriate RNA dye selection and concentration is critical for accurate assessment of RNA loading [22]. SYBR Gold at manufacturer-suggested dilution (1:10,000) shows poor discrimination of different RNA loading amounts, potentially leading to erroneous conclusions about sample equal loading [22].
Cell-Type Specific Optimization: Different cell types exhibit varying glycoRNA levels and tolerance to metabolic labeling reagents [22]. Optimization of Ac4ManNAz treatment duration and RNA loading amounts is recommended for different experimental systems [22].
GlycoRNAs represent a paradigm-shifting discovery in molecular biology, establishing RNA as a third class of glycoconjugate alongside glycoproteins and glycolipids. Their primary functions in immune recognition and intercellular communication position them as significant players in physiological and pathological processes. The role of glycoRNAs as ligands for Siglec receptors and mediators of neutrophil recruitment highlights their importance in immune regulation, while their presence on extracellular vesicles extends their communicative reach throughout biological systems.
Advanced detection methodologies, including drFRET, rPAL, ARPLA, and Clier-qPCR, have enabled increasingly sensitive and specific analysis of glycoRNA composition and function. The inverse relationship between glycoRNA expression and tumor aggressiveness, coupled with their exceptional diagnostic performance in cancer detection, underscores their translational potential.
As research methodologies continue to evolve and address current technical challenges, glycoRNAs are poised to become increasingly important targets for therapeutic intervention and biomarker development across a spectrum of human diseases. Their unique position at the interface of RNA biology and glycobiology offers exciting opportunities for scientific discovery and clinical innovation.
Tools and Techniques: Profiling GlycoRNA for Diagnostic and Therapeutic Innovation
The discovery of glycoRNAs—small, non-coding RNAs modified with sialylated glycans on the cell surface—required a paradigm shift in glycosylation biology. This whitepaper details the core methodology that enabled this breakthrough: metabolic labeling with bioorthogonal chemical reporters followed by click chemistry-mediated detection. We provide an in-depth technical guide to this foundational toolkit, outlining the specific reagents, experimental protocols, and key quantitative data that researchers can use to investigate glycoRNAs. Furthermore, we contextualize this methodology within the broader glycoRNA research landscape, discussing subsequent validation studies, emerging controversies regarding isolation artifacts, and advanced techniques that are expanding the field's capabilities.
For decades, glycosylation was considered a modification exclusive to proteins and lipids. The landmark 2021 discovery by Flynn et al. identified a third class of glycosylated molecules: glycoRNAs [1] [24] [25]. These are small, non-coding RNAs (such as Y RNAs, tRNAs, and snRNAs) covalently modified with sialylated N-glycans and displayed on the outer surface of mammalian cells [1] [25]. Their surface localization and glycan composition allow them to interact with immunoregulatory receptors like Siglecs, positioning them as potential novel players in cell-cell communication and immune signaling [1] [14] [24].
The initial detection of these molecules posed a significant technical challenge, as they were unexpected and existed at the interface of two traditionally separate fields: RNA biology and glycobiology. The solution was the strategic combination of metabolic labeling and click chemistry, a toolkit that allows for the specific tagging and isolation of newly synthesized glycoRNAs from complex cellular environments.
The Core Methodology: Metabolic Labeling and Click Chemistry
This section details the experimental workflow, from cell culture to detection, providing a protocol for researchers to implement in their studies.
The following diagram illustrates the sequential steps involved in the metabolic labeling and click chemistry workflow for glycoRNA detection.
Key Research Reagents and Their Functions
The experimental workflow relies on specific, critical reagents, each serving a distinct function to ensure specific labeling and detection.
Table 1: Essential Reagents for Metabolic Labeling and Click Chemistry of GlycoRNAs
| Reagent | Function | Key Characteristics |
|---|---|---|
| Acâ‚„ManNAz (N-azidoacetylmannosamine-tetraacylated) [26] [27] [24] | Metabolic precursor for sialic acid. Cells incorporate it into nascent N-glycans on glycoRNAs, introducing a chemical handle (azide group). | Peracetylated form enhances cell permeability. Serves as a "metabolic chemical reporter." |
| DBCO-Biotin (Dibenzocyclooctyne-Biotin) [26] [27] | Click chemistry reagent. Its DBCO group reacts specifically with the azide on metabolically labeled glycans, conjugating a biotin tag to the glycoRNA. | Copper-free cycloaddition avoids metal-induced RNA degradation. Biotin enables high-affinity capture/detection. |
| Streptavidin-HRP Probe [27] | Detection agent. Binds with high specificity and sensitivity to the biotin tag on glycoRNAs. | Used in conjunction with northern blotting to visualize glycoRNA signals. |
| TRIzol Reagent [9] | RNA isolation. Enables extraction of total RNA, including glycoRNAs, from cells. | Provides a denaturing environment that helps preserve the integrity of the RNA-glycan linkage. |
| Silica Spin Columns [9] | RNA purification. Used post-extraction to desalt RNA and remove unconjugated click chemistry reagents. | Critical for reducing background signal in downstream detection. |
Detailed Experimental Protocol
- Metabolic Labeling: Culture cells (e.g., HeLa, NIH3T3) in medium supplemented with 100 µM Ac₄ManNAz for 36-40 hours [26] [9]. This allows cells to metabolically convert Ac₄ManNAz into N-azido sialic acid (Neu5Az) and incorporate it into the sialylated N-glycans of glycoRNAs.
- RNA Extraction and Purification: Lyse cells and extract total RNA using a standard TRIzol/chloroform protocol [9]. Following extraction, further purify the RNA using silica-based spin columns to remove metabolites and salts that could interfere with the subsequent click reaction [9].
- Click Chemistry Conjugation: React the purified RNA with DBCO-PEG4-Biotin (e.g., 25 °C incubation) [26]. This copper-free, strain-promoted azide-alkyne cycloaddition (SPAAC) covalently links the biotin tag specifically to the azide-containing glycans on glycoRNAs.
- Post-Click Purification: After the click reaction, perform another silica column purification to remove excess, unreacted DBCO-biotin, which is crucial for minimizing false-positive signals in detection [9].
- Detection via Northwestern Blotting:
- Separate the biotinylated RNA species using denaturing gel electrophoresis.
- Transfer the RNA to a membrane.
- Probe the membrane with a streptavidin-horseradish peroxidase (HRP) conjugate.
- Visualize using chemiluminescence. The presence of high-molecular-weight biotin signals (>10 kb) in the small RNA fraction (<200 nucleotides) indicates the presence of glycoRNAs [1] [24].
Key Validation and Controls
The initial studies established the specificity of this toolkit through rigorous controls, summarized in the table below.
Table 2: Key Experimental Controls for Validating Genuine GlycoRNA Signals
| Control Experiment | Procedure | Expected Result for Authentic GlycoRNA |
|---|---|---|
| No Metabolic Labeling | Omit Acâ‚„ManNAz from culture medium. | Absence of biotin signal on northwestern blot. Confirms signal is from incorporated label [26]. |
| RNase Treatment | Treat purified, biotinylated RNA samples with RNase A/T1. | Loss of biotin signal. Confirms the signal is associated with an RNA molecule [1] [9]. |
| Enzymatic Deglycosylation | Treat samples with PNGase F, an enzyme that cleaves N-glycans. | Attenuation of signal. Confirms the presence of an N-glycan structure [1] [25]. |
| Disruption of Glycosylation Machinery | Use genetic (e.g., CRISPR knockout of OST complex subunits) or pharmacological (e.g., NGI-1) inhibition of N-glycosylation. | Significant reduction in glycoRNA production. Confirms dependence on canonical glycosylation pathways [1] [27] [25]. |
Context and Evolution of the Field
The discovery toolkit has served as a springboard for deeper investigation, leading to mechanistic insights, functional discoveries, and methodological debates.
Beyond the Initial Discovery: Mechanism and Function
Subsequent research has built upon this foundation:
- The Glycan-RNA Linkage: A pivotal 2024 study used RNA-optimized periodate oxidation and aldehyde ligation (rPAL) and mass spectrometry to identify 3-(3-amino-3-carboxypropyl)uridine (acp3U), a modified uridine, as the primary site for N-glycan attachment on RNA [1] [24] [25].
- Functional Roles: GlycoRNAs have been shown to act as ligands for members of the Siglec family (e.g., Siglec-11) [1] and for P-selectin on endothelial cells, where they enhance neutrophil recruitment to inflammatory sites [1] [26] [28]. Their presence on the surface of cancer cells and small extracellular vesicles (sEVs) also suggests potential roles as diagnostic biomarkers and in tumor immunology [1] [26] [28].
A Note on Controversy and Technical Refinement
The field has actively engaged in self-critical evaluation to ensure robustness. A key study highlighted a potential source of artifact: certain glycoproteins (e.g., LAMP1) can co-purify with small RNA preparations using the standard protocol [9]. These contaminating glycoproteins are resistant to mild proteinase K treatment but are degraded under denaturing conditions. This finding does not invalidate glycoRNAs but emphasizes the critical need for rigorous controls, including:
- Denaturing Proteinase K Treatment: Using buffers containing SDS to ensure complete protein digestion [9].
- Orthogonal Validation: Employing newer, independent methods like rPAL [1] [27] or ARPLA [1] [27] that do not rely on metabolic labeling to confirm results.
The Expanding Scientist's Toolkit
While metabolic labeling and click chemistry were instrumental for discovery, the field has since developed a more diverse arsenal of techniques.
Table 3: Evolution of Key Detection and Analysis Methods for GlycoRNA
| Method | Principle | Advantages |
|---|---|---|
| rPAL (RNA-optimized periodate oxidation and aldehyde ligation) [1] [27] | Uses periodate to oxidize sialic acid diols on native glycoRNAs, enabling biotin tagging without metabolic labeling. | ~25x more sensitive than metabolic labeling; works on native structures [24]. |
| ARPLA (Aptamer and RNA in-situ hybridization-mediated Proximity Ligation Assay) [1] [27] | Uses dual probes for glycan and RNA sequence, triggering a rolling circle amplification for visualization. | Enables high-sensitivity, high-selectivity spatial imaging at the single-cell level [1]. |
| drFRET (Dual-recognition FRET) [26] | Uses dual DNA probes (for glycan and RNA) to generate a FRET signal only when both are in proximity. | Allows ultrasensitive profiling of glycoRNAs on sEVs from minimal biofluids (e.g., 10 µL); high clinical potential [26]. |
| Lectin Affinity Purification [27] | Uses lectins (e.g., WGA, MALII) that bind specifically to sialylated glycans to enrich glycoRNAs. | Effective for isolation and enrichment of glycosylated RNAs from total RNA samples. |
| Isodiospyrin | Isodiospyrin, CAS:20175-84-2, MF:C22H14O6, MW:374.3 g/mol | Chemical Reagent |
| (6-Fluoropyridin-3-yl)methanamine | (6-Fluoropyridin-3-yl)methanamine, CAS:205744-17-8, MF:C6H7FN2, MW:126.13 g/mol | Chemical Reagent |
Metabolic labeling coupled with click chemistry formed the essential, foundational toolkit that unveiled the existence of glycoRNAs, challenging long-held dogmas in molecular biology. The detailed protocols and reagent information provided in this whitepaper offer a roadmap for researchers to conduct initial investigations in this emerging field. As the science progresses, this initial toolkit is being supplemented and validated by a new generation of detection methods (rPAL, ARPLA, drFRET) that offer greater sensitivity, spatial resolution, and clinical applicability. For scientists entering this field, a rigorous approach that incorporates robust controls to account for potential artifacts is paramount. The continued exploration of glycoRNAs, powered by an ever-improving toolkit, holds significant promise for advancing our understanding of immune regulation, cancer biology, and the development of novel diagnostic and therapeutic strategies.
GlycoRNAs represent a groundbreaking discovery in molecular biology, defined as small non-coding RNAs covalently modified with sialylated glycans [6]. This novel class of biomolecules, discovered in 2021, challenges long-standing paradigms by establishing RNA as a third target for glycosylation alongside proteins and lipids [6] [24]. GlycoRNAs are primarily displayed on the cell surface where they function as ligands for immune receptors such as Siglec family members, positioning them as key players in cell-to-cell communication and immune regulation [6] [1] [25].
The precise characterization of these molecules has proven challenging due to their low abundance and the technical limitations of initial detection methods. Early approaches relying on metabolic labeling with unnatural sugars provided the first evidence for glycoRNAs' existence but lacked the sensitivity for comprehensive analysis [24]. This sensitivity gap significantly hampered investigations into the biological roles and molecular structures of glycoRNAs, creating an urgent need for advanced enrichment and detection strategies capable of probing this new frontier in RNA biology.
The rPAL Method: Principles and Advantages
RNA-optimized periodate oxidation and aldehyde ligation (rPAL) represents a significant technological advancement specifically designed to address the limitations of previous glycoRNA detection methods. This innovative technique leverages the unique chemical properties of glycan moieties to enable highly sensitive and specific identification of glycoRNAs [1].
The fundamental principle underlying rPAL is the specific oxidation of 1,2-diols present in the sialic acid components of glycans. Periodate treatment cleaves these diols to generate reactive aldehyde groups, which subsequently form stable oxime bonds with aminooxy-functionalized solid-phase supports [1]. This chemical strategy allows for specific labeling and efficient capture of glycoRNAs from complex biological mixtures.
Compared to earlier metabolic labeling techniques, rPAL provides a dramatic ~25-fold increase in sensitivity and significantly improved signal recovery [24]. This enhanced performance has proven crucial for advancing the field, particularly in identifying the precise molecular linkage between glycans and their RNA substrates. The method's superior sensitivity enables researchers to work with smaller sample volumes while obtaining more comprehensive data, making it possible to explore glycoRNA biology in greater depth than previously achievable.
Table 1: Comparison of GlycoRNA Detection Methods
| Method | Principle | Sensitivity | Key Applications |
|---|---|---|---|
| Metabolic Labeling + Northwestern Blot | Incorporation of clickable azido-sialic acid into glycans [6] [24] | Lower | Initial discovery, validation of cell-surface localization |
| rPAL (RNA-optimized periodate oxidation and aldehyde ligation) | Periodate oxidation of sialic acid diols followed by aldehyde ligation [1] [24] | ~25-fold higher than metabolic labeling | High-sensitivity enrichment, structural characterization, identification of acp3U linkage |
| drFRET | Dual recognition of glycans and RNA via fluorescence resonance energy transfer [1] [24] | High (works with 10 µL biofluid) | Clinical diagnostics, extracellular vesicle profiling, cancer subclassification |
| ARPLA | Dual recognition of glycans and RNA triggering proximity ligation [1] | Single-cell resolution | Spatial visualization, intracellular trafficking studies |
Detailed rPAL Experimental Workflow
The successful implementation of rPAL involves a series of carefully optimized steps designed to preserve the integrity of glycoRNA molecules while maximizing enrichment efficiency. The following protocol outlines the key stages of this process, incorporating critical considerations for experimental success.
Sample Preparation and RNA Extraction
- Starting Material: Begin with cultured mammalian cells (e.g., HeLa, NIH3T3) grown to appropriate confluence [9].
- Cell Lysis: Use TRIzol reagent for complete cell lysis and RNA stabilization. Incubate lysate at 37°C for 10 minutes to enhance lysis efficiency [9].
- Phase Separation: Add 0.2 volumes of chloroform to the homogenized lysate, mix thoroughly, and centrifuge at 4,000g for 10 minutes [9].
- RNA Precipitation: Transfer the aqueous phase to a fresh tube and mix with 1.1 volumes of 100% isopropanol. Precipitate at -20°C for 1 hour [9].
- RNA Pellet Collection: Centrifuge at 4,000g for 2 hours at 4°C to pellet RNA. Wash with 80% ethanol, air-dry, and solubilize in ultrapure water overnight [9].
rPAL-Specific Processing Steps
- Periodate Oxidation: Treat purified RNA with sodium periodate to oxidize vicinal diols in sialic acid residues, generating reactive aldehyde groups [1].
- Aldehyde Ligation: Incubate oxidized RNA with aminooxy-functionalized solid-phase supports to form stable oxime bonds with glycoRNAs [1].
- Stringent Washing: Apply rigorous washing conditions to remove non-specifically bound RNAs while retaining covalently linked glycoRNAs.
- Elution: Release captured glycoRNAs from solid supports using appropriate elution buffers compatible with downstream applications.
Downstream Analysis Options
- Small RNA Sequencing: Use platforms like NEXTFLEX Small RNA Sequencing Kit V4 to identify glycosylated RNA species [24].
- Mass Spectrometry: Employ SWATH-MS approaches to characterize glycan composition and attachment sites [25].
- Validation Experiments: Implement orthogonal methods such as northern blotting or drFRET to confirm results [24].
Key Applications and Discoveries Enabled by rPAL
The implementation of rPAL technology has catalyzed significant advances in glycoRNA research, enabling discoveries that were previously beyond methodological reach. These breakthroughs have substantially expanded our understanding of glycoRNA biology and opened new avenues for therapeutic development.
Most notably, the application of rPAL coupled with high-sensitivity mass spectrometry led to the identification of 3-(3-amino-3-carboxypropyl)uridine (acp3U) as the primary nucleotide anchoring site for N-glycan attachment [1] [25]. This discovery resolved a fundamental question regarding the chemical nature of the RNA-glycan linkage and revealed that a highly conserved modified uridine present in both bacterial and mammalian tRNAs serves as the critical attachment point [1]. The acp3U modification was previously known to enhance tRNA thermostability and play significant roles in cellular physiology, but its function as a glycan attachment site was entirely novel [1].
Beyond structural insights, rPAL has facilitated the investigation of glycoRNA functions in physiological and pathological contexts. The technology has enabled researchers to profile glycoRNA expression patterns across different tissues and cell types, revealing that glycoRNA glycans differ significantly in composition from protein-bound glycans and exhibit tissue-specific abundance patterns [24]. These findings suggest that glycoRNA levels vary based on cellular context and physiological state, supporting their potential roles as dynamic regulators of cellular communication rather than static structural components.
In the diagnostic realm, rPAL-enhanced detection has contributed to the development of sensitive assays for disease detection. When combined with complementary approaches like drFRET, glycoRNA profiling has demonstrated remarkable diagnostic performance, with studies reporting 100% accuracy distinguishing cancer versus control samples and approximately 90% accuracy in subclassifying cancer types within patient cohorts [24]. These clinical applications highlight the translational potential of glycoRNA research and underscore the importance of sensitive detection methods like rPAL in unlocking this potential.
Table 2: Key Research Reagent Solutions for GlycoRNA Studies
| Reagent/Category | Specific Examples | Function/Application |
|---|---|---|
| Metabolic Labeling Reagents | Acâ‚„ManNAz (peracetylated N-azidoacetylmannosamine) [9] [24] | Incorporation of clickable azido-sialic acid into nascent glycans for detection |
| Enrichment Tools | Aminooxy-functionalized solid supports, Lectins (e.g., WGA) [1] [24] | Specific capture of glycoRNAs based on glycan properties |
| Enzymatic Tools | PNGase F, Proteinase K, RNase A/T1 [6] [9] | Characterization of glycoRNA sensitivity and composition |
| Detection Probes | Northern blot probes, drFRET dual-recognition probes [1] [24] | Visualization and quantification of glycoRNAs |
| Sequencing Kits | NEXTFLEX Small RNA Sequencing Kit V4 [24] | Identification of RNA sequences carrying glycan modifications |
| Mass Spectrometry Standards | GlycanDIA workflow components [24] | Quantitative glycomic analysis of glycoRNA modifications |
Critical Considerations and Methodological Challenges
While rPAL represents a significant advancement in glycoRNA research, several methodological challenges and considerations remain essential for proper experimental design and data interpretation. Awareness of these factors ensures the appropriate application of the technology and validation of findings.
A primary consideration involves the potential for co-purification of glycoproteins during RNA extraction procedures. Recent investigations have demonstrated that glycoproteins such as LAMP1 can persist through standard glycoRNA isolation protocols, with glycosylated molecules showing resistance to RNase A/T1 treatment but sensitivity to proteinase K digestion under denaturing conditions [9]. This observation highlights the importance of incorporating rigorous controls, including proteinase K treatment with denaturation, to distinguish authentic glycoRNAs from glycoprotein contaminants.
The specificity of periodate oxidation also warrants careful consideration. While rPAL leverages periodate's reactivity with 1,2-diols in sialic acids, other biomolecules containing similar chemical motifs could potentially be captured. Implementing appropriate control experiments without periodate treatment helps establish background binding levels and validates the specificity of enrichment.
Additionally, the efficiency of oxime bond formation between oxidized glycans and aminooxy-functionalized supports can vary based on reaction conditions such as pH, temperature, and catalyst presence. Optimizing these parameters for specific experimental systems maximizes capture efficiency while minimizing non-specific interactions.
Finally, the downstream compatibility of rPAL-enriched samples with various analytical platforms requires assessment. While rPAL-enriched glycoRNAs are suitable for sequencing and mass spectrometric analysis, the chemical modifications introduced during the procedure may influence certain applications. Researchers should validate their specific analytical workflows to ensure compatibility with rPAL-processed samples.
The development and implementation of rPAL technology marks a transformative advancement in the field of glycoRNA research, providing the sensitivity and specificity necessary to probe this newly discovered category of biomolecules. The method's capacity to enable precise enrichment and characterization of glycoRNAs has already yielded fundamental insights, most notably the identification of acp3U as the glycan attachment site, while simultaneously opening new avenues of investigation into glycoRNA biology and function.
Looking forward, further refinements to the rPAL methodology will likely focus on enhancing throughput, reducing sample requirements, and expanding compatibility with emerging analytical platforms. The integration of rPAL with single-cell sequencing technologies represents a particularly promising direction, potentially enabling the exploration of glycoRNA heterogeneity at cellular resolution. Additionally, adaptations of the core rPAL principle may facilitate in situ mapping of glycoRNAs within tissues, providing spatial context to complement molecular characterization.
As these methodological advances continue to mature, rPAL and related technologies will play increasingly vital roles in elucidating the pathological significance of glycoRNAs in human diseases. The demonstrated involvement of glycoRNAs in immune recognition pathways and their emerging potential as diagnostic biomarkers highlight the translational importance of these investigations [6] [1] [24]. With advanced enrichment strategies like rPAL providing the necessary technical foundation, the field is positioned to make rapid progress in understanding glycoRNA functions and leveraging this knowledge for therapeutic benefit. The ongoing integration of glycobiology and RNA biology through tools like rPAL promises to reveal new dimensions of cellular regulation and communication, fundamentally expanding our understanding of molecular biology while creating novel opportunities for biomedical intervention.
Glycosylated RNA (glycoRNA) represents a paradigm shift in molecular biology, emerging as a previously unknown class of biomolecules where RNA is modified with glycans. Traditionally, glycosylation was considered a modification exclusive to proteins and lipids, with RNA biology and glycobiology viewed as two distinct fields—the former confined to the nucleus and cytoplasm, and the latter localized to the endoplasmic reticulum-Golgi system [1]. This discovery has fundamentally expanded our understanding of the cellular machinery, revealing that small non-coding RNAs can carry N-glycan structures rich in sialic acid and fucose components and exist on the cell surface [1]. These glycoRNAs have been confirmed as potential ligands for the sialic acid-binding immunoglobulin-like lectin (Siglec) family of receptors, which are implicated in immunoregulation and tumor immune evasion [1]. This positions glycoRNAs as potential key players in intercellular communication and immune recognition processes [1]. However, progress in understanding their precise biological roles has been hampered by a lack of tools for direct visualization within native cellular contexts. This whitepaper details a groundbreaking methodological solution—the Sialic Acid Aptamer and RNA in situ Hybridization-mediated Proximity Ligation Assay (ARPLA)—which enables high-resolution spatial imaging of glycoRNAs in single cells, thereby opening new avenues for research and therapeutic development [29] [30].
The Critical Need for Advanced GlycoRNA Imaging
Prior to the development of ARPLA, researchers relied on a patchwork of techniques to study glycoRNAs. Metabolic labeling combined with click chemistry and RNA blotting confirmed their existence, while next-generation sequencing allowed for profiling of glycoRNA sequences [29]. Mass spectrometry helped analyze glycan composition, and antibodies or lectins were used for initial imaging attempts [29] [31]. However, these methods collectively suffered from significant limitations in specificity, spatial resolution, and the ability for direct visualization on cell surfaces [31] [30]. Methods using antibodies or glycan-binding proteins, in particular, lacked the selectivity required to unequivocally distinguish the unique RNA-glycan conjugate from other glycosylated molecules or unmodified RNAs in close proximity [29] [31]. This lack of effective visualization tools has left fundamental questions about glycoRNA biology largely unanswered, including their precise subcellular distribution, trafficking mechanisms, and functional interactions at the cell membrane.
Table 1: Comparison of GlycoRNA Detection Methods
| Method | Key Principle | Advantages | Limitations |
|---|---|---|---|
| Metabolic Labeling & RNA Blot [29] | Incorporation of clickable sugars into glycans, followed by biotinylation and detection. | Confirms existence of glycoRNAs. | Lacks spatial and sequence information. |
| Next-Generation Sequencing [29] | Enrichment and sequencing of glycoRNAs. | Profiles glycoRNA sequences. | Does not provide spatial context or localization. |
| Mass Spectrometry [29] | Analysis of glycan composition and conformation. | Reveals glycan structure. | Requires sample disruption, no imaging capability. |
| Antibody/Lectin Imaging [31] | Use of antibodies (e.g., against dsRNA) or lectins to bind targets. | Can verify surface presence. | Lacks selectivity and specificity for the glycoRNA molecule. |
| ARPLA [29] [30] | Dual recognition of glycan and RNA moieties via proximity ligation. | High sensitivity & selectivity; provides sequence-specific spatial information. | Protocol complexity. |
Furthermore, the field has recently been challenged by findings suggesting that current glycoRNA isolation protocols may co-purify glycoproteins, which could represent a considerable source of glycans in glycoRNA samples and potentially lead to artifacts [9]. This underscores the pressing need for highly specific in situ detection methods like ARPLA, which can visualize glycoRNAs within their native cellular environment without requiring extensive biochemical purification.
ARPLA: Core Technology and Mechanism
The Sialic Acid Aptamer and RNA in situ Hybridization-mediated Proximity Ligation Assay (ARPLA) is a novel imaging technology designed to directly visualize glycoRNAs with high sensitivity and selectivity [29]. Its power derives from a dual-recognition mechanism that specifically targets the unique glycan-RNA conjugate, providing both sequence and spatial information that was previously inaccessible [30].
The ARPLA Workflow
The ARPLA procedure involves a meticulously orchestrated four-step process, as illustrated in the diagram below.
Step 1: Dual Probe Binding. Two probes are simultaneously applied to the cell sample. The glycan probe features a sialic acid (Neu5Ac) aptamer—a single-stranded nucleic acid with high affinity (Kd ≈ 91 nM) for the glycan moiety—a spacer to prevent steric hindrance, and a DNA linker (Linker G) [29]. In parallel, the RNA-binding probe contains a DNA strand for RNA in situ hybridization (RISH) targeting a specific RNA sequence (e.g., U1 RNA), a spacer, and a second DNA linker (Linker R) [29].
Step 2: Connector Hybridization and Proximity Ligation. If and only if both probes are bound in close proximity to the same glycoRNA molecule, two connector oligonucleotides hybridize to Linker G and Linker R, bringing their ends together. This proximity enables an enzymatic ligation reaction that joins the connectors into a single, circularized DNA molecule [29].
Step 3: Rolling Circle Amplification (RCA). The circularized DNA serves as a template for RCA, an enzymatic process that generates a long, single-stranded DNA concatemer containing hundreds of repeats complementary to the circle [29]. This dramatic amplification is key to achieving high detection sensitivity.
Step 4: Fluorescent Detection. Fluorophore-labeled oligonucleotide probes are hybridized to the repetitive RCA product. This results in a bright, localized fluorescent signal that pinpoints the spatial location of the target glycoRNA, which can be visualized using confocal laser-scanning microscopy [29].
Key Technological Innovations
ARPLA's design incorporates several critical innovations that set it apart from previous methods:
- Dual Recognition and Specificity: The core innovation is the requirement for dual binding events in close proximity. This drastically reduces false-positive signals that could arise from detecting free glycans or unmodified RNAs individually, or from two unrelated molecules that are far apart [29] [30]. The use of a high-affinity Neu5Ac aptamer provides a more specific targeting mechanism than broader-binding lectins [29].
- Signal Amplification: The integration of proximity ligation with RCA transforms a single binding event into a massive, localized signal, enabling the detection of low-abundance glycoRNAs that would otherwise be undetectable [29].
- Sequence Specificity: Unlike methods that only target the glycan, the RISH component of ARPLA allows researchers to investigate glycoRNAs with specific RNA sequences, enabling more precise functional studies [29] [30].
Experimental Validation and Key Protocols
The performance and specificity of ARPLA have been rigorously validated through a series of controlled experiments in various cell models.
Specificity and Selectivity Controls
To confirm that ARPLA signals genuinely represent glycoRNAs, researchers performed essential control experiments and enzymatic treatments [29]:
- Component Omission: Omitting any single core component—the aptamer, the RISH probe, or the connectors—led to a dramatic reduction (9- to 270-fold) in fluorescence signal [29].
- Scrambled Aptamer Control: Replacing the specific Neu5Ac aptamer with a scrambled DNA sequence reduced signals by tenfold, confirming the importance of specific glycan recognition [29].
- RNase Treatment: Digesting the RNA moiety with RNase A or RNase T1 reduced signals by approximately 90%, demonstrating dependence on the RNA component [29].
- Glycosylation Inhibition: Treating cells with glycosylation inhibitors (NGI-1, kifunensine, swainsonine) or specific glycosidases (PNGase-F, neuraminidase A) reduced ARPLA signals by 86-93%, confirming dependence on the glycan moiety [29].
Table 2: Summary of ARPLA Validation Experiments and Outcomes
| Experimental Condition | Targeted Moiety | Key Outcome | Interpretation |
|---|---|---|---|
| Omit Aptamer [29] | Glycan | 13-fold signal decrease | Signal requires glycan binding. |
| Omit RISH Probe [29] | RNA | 270-fold signal decrease | Signal requires RNA binding. |
| Use Scrambled Aptamer [29] | Glycan | 10-fold signal decrease | Signal requires specific glycan recognition. |
| RNase A/T1 Treatment [29] | RNA | ~90% signal decrease | Signal is RNA-dependent. |
| Glycosylation Inhibitors [29] | Glycan | ~90% signal decrease | Signal is glycan-dependent. |
| PNGase-F/Neuraminidase A [29] | Glycan | ~90% signal decrease | Signal is specific to N-glycan/sialic acid. |
Key Experimental Protocol for ARPLA Imaging
The following is a generalized protocol for implementing ARPLA, as derived from the methodology described in the research [29]:
- Cell Preparation and Fixation: Culture cells on appropriate imaging-compatible surfaces (e.g., glass coverslips). For cell surface glycoRNA imaging, fix cells with paraformaldehyde without permeabilization to preserve membrane integrity.
- Dual Probe Hybridization: Incubate fixed cells with a hybridization mixture containing both the glycan probe (e.g., Neu5Ac aptamer-linker G construct) and the RNA-binding probe (RISH probe-linker R construct). The RISH probe is designed to be complementary to the target RNA sequence (e.g., U1 snRNA).
- Proximity Ligation and RCA: After washing away unbound probes, add the connector oligonucleotides and ligase to the cells. If the two probes are bound in close proximity on a glycoRNA molecule, the connectors will hybridize and be ligated into a circle. Then, add DNA polymerase to initiate RCA, which amplifies the circular template into a long, single-stranded DNA product.
- Fluorescent Detection and Imaging: Hybridize fluorophore-labeled detection oligonucleotides to the repetitive RCA product. Wash the sample to remove unbound reporters and mount for microscopy. Acquire images using a confocal laser-scanning microscope, using appropriate laser lines and filters for the chosen fluorophore.
Biological Insights Enabled by ARPLA
The application of ARPLA has yielded several significant discoveries regarding the biology of glycoRNAs, moving beyond mere detection to functional insights.
- Spatial Distribution and Colocalization: ARPLA has confirmed the presence of glycoRNAs on the plasma membrane and revealed their colocalization with lipid rafts, suggesting an association with these specialized membrane microdomains known for organizing signaling molecules [29].
- Intracellular Trafficking: The technology has enabled the observation of intracellular glycoRNAs, and evidence suggests they traffic to the cell surface through SNARE protein-mediated secretory exocytosis, a pathway also used for transporting vesicles and secreting neurotransmitters [29].
- Role in Cancer and Immunity: Functional studies using ARPLA have linked glycoRNA abundance to disease states. In breast cancer models, surface glycoRNA levels were inversely associated with tumor malignancy and metastasis [29] [30]. In immune models, glycoRNAs appear to mediate monocyte-endothelial cell interactions, suggesting a role in inflammatory responses [29]. Furthermore, glycoRNAs can bind to Siglec receptors, implicating them in the regulation of immune cell functions [1].
The Scientist's Toolkit: Essential Reagents for ARPLA
Implementing ARPLA requires a set of specific, high-quality reagents. The table below details the core components and their functions.
Table 3: Key Research Reagent Solutions for ARPLA
| Reagent / Component | Function / Description | Key Feature / Note |
|---|---|---|
| Sialic Acid Aptamer [29] | Binds specifically to the N-acetylneuraminic acid (sialic acid) on the glycan moiety of glycoRNA. | High-affinity (Kd ~91 nM); more specific than lectins. |
| RISH DNA Probe [29] | Hybridizes to the specific RNA sequence of the target glycoRNA (e.g., U1, Y RNA). | Provides sequence specificity; can be customized. |
| Connector Oligonucleotides [29] | Short DNA strands that hybridize to linkers on the primary probes and are ligated to form a circle. | Enables proximity-dependent circularization. |
| Ligase Enzyme [29] | Enzymatically joins the ends of the connector oligonucleotides upon hybridization. | Critical for the proximity ligation step. |
| DNA Polymerase [29] | Performs Rolling Circle Amplification (RCA) using the circular DNA as a template. | Generates the amplified signal for detection. |
| Fluorophore-labeled Oligonucleotides [29] | Detection probes that bind to the repetitive RCA product, generating a fluorescent signal. | Allows visualization via fluorescence microscopy. |
| Deoxylapachol | Deoxylapachol Research Compound|Naphthoquinone | High-purity Deoxylapachol, a natural naphthoquinone from teak wood. For Research Use Only (RUO). Not for human, veterinary, or household use. |
| trans-3-(Trimethylsilyl)allyl alcohol | trans-3-(Trimethylsilyl)allyl alcohol, CAS:59376-64-6, MF:C6H14OSi, MW:130.26 g/mol | Chemical Reagent |
ARPLA represents a significant leap forward for the field of glycoRNA biology. By enabling the high-sensitivity, high-specificity spatial imaging of these elusive molecules in single cells, it provides a powerful tool to uncover their fundamental biological roles, trafficking mechanisms, and interactions at the cell surface [29] [30]. The initial insights gained into their involvement in cancer progression and immune responses highlight the potential of glycoRNAs as new biomarkers and therapeutic targets [29] [1]. As the technology sees wider adoption and potentially combines with other spatial transcriptomic methods [32], it is poised to accelerate our understanding of this novel layer of molecular regulation, paving the way for innovations in drug discovery and diagnostic applications, particularly within the rapidly expanding RNA therapeutics market [30].
Glycosylated RNA (glycoRNA) represents a groundbreaking discovery in molecular biology, establishing RNA as a third class of glycoconjugate alongside glycoproteins and glycolipids [1]. These molecules are predominantly small non-coding RNAs modified with N-glycans rich in sialic acid and fucose, and they have been confirmed to exist on the cell surface, suggesting pivotal roles in intercellular communication and immune recognition processes [1]. The unique subcellular localization and molecular characteristics of glycoRNAs position them as potential mediators of immune regulation through interactions with Siglec family receptors, and their expression patterns are increasingly linked to disease states, including cancer [1] [14].
However, the study of glycoRNAs presents unique analytical challenges. These molecules account for only a tiny fraction of the total transcriptome, are present in extremely low abundance, and conventional RNA sequencing methods cannot distinguish glycosylated from non-glycosylated RNA species [33] [34] [35]. To overcome these limitations, researchers have developed Clier-seq (Click chemistry-based enrichment of glycoRNAs sequencing), a specialized pipeline designed for the transcriptome-wide identification and characterization of glycoRNAs with high specificity and sensitivity [33] [35].
Clier-seq: Core Methodology and Workflow
Clier-seq integrates metabolic labeling, bioorthogonal chemistry, and high-throughput sequencing to specifically isolate and sequence glycosylated RNAs. The method was explicitly designed to maximize coverage of glycoRNAs ranging from 50 to 2,000 nucleotides, capturing both canonical and novel glycoRNA subtypes that were previously undetectable [33] [35].
Metabolic Labeling and Chemical Tagging
The initial stage of Clier-seq involves introducing specific chemical tags into glycoRNAs through metabolic engineering:
- Ac4ManNAz Labeling: Living cells are cultured with peracetylated N-azidoacetylmannosamine (Ac4ManNAz), an azide-modified mannose analog that is metabolically incorporated into sialic acid residues of glycoRNAs [34] [36]. This incubation typically occurs over 48 hours to ensure sufficient incorporation [34].
- RNA Extraction and Purification: Following metabolic labeling, total RNA is extracted using Trizol lysis, followed by high-concentration proteinase K digestion to remove potentially contaminating glycoproteins, and rigorous purification via silica columns [34].
- Click Chemistry Conjugation: The azide groups incorporated into glycoRNAs undergo a copper-free click chemistry reaction with DBCO-PEG4-biotin, resulting in biotin tagging specifically of glycosylated RNAs [34]. This bioorthogonal approach minimizes damage to RNA molecules while ensuring high specificity.
Enrichment and Library Preparation
The critical enrichment phase separates glycoRNAs from the total RNA pool:
- Affinity Purification: Biotinylated glycoRNAs are isolated using streptavidin magnetic beads through stringent pull-down assays [34]. Multiple washing steps with appropriate buffers remove non-specifically bound RNAs.
- Quality Control and Validation: Enriched glycoRNAs undergo rigorous QC assessment, and specificity is often confirmed through Clier-qPCR assays (click chemistry-based enrichment of glycoRNAs RT-qPCR) [33] [35].
- Library Construction: Library preparation is optimized for the unique characteristics of glycoRNAs, typically targeting small non-coding RNAs with modifications to standard adapters and reverse transcription protocols to accommodate glycan presence [36].
Sequencing and Bioinformatics Analysis
The final stage involves sequencing and specialized computational analysis:
- High-Throughput Sequencing: Libraries are sequenced using platforms such as Illumina SBS to achieve sufficient depth for low-abundance glycoRNAs [34] [36].
- Bioinformatics Pipeline: The HISAT-StringTie-Ballgown pipeline is employed for read alignment, transcript assembly, and expression quantification [33] [35]. Novel glycoRNA subtypes are predicted through specialized computational approaches.
- Glycosylation Site Analysis: Advanced tools, including sequence motif searches and machine learning approaches (e.g., GlyinsRNA with AUROC ≈ 0.79), help predict glycosylation sites [36].
Table 1: Key Reagents and Solutions for Clier-seq Experiments
| Reagent/Solution | Function | Application Notes |
|---|---|---|
| Ac4ManNAz | Metabolic precursor for azide-modified sialic acids | Incorporated during cell culture (typically 48 hours) [34] |
| DBCO-PEG4-Biotin | Bioorthogonal reagent for click chemistry | Conjugates with azide tags for streptavidin pull-down [34] |
| Streptavidin Magnetic Beads | Affinity capture of biotinylated glycoRNAs | Enable specific enrichment from complex RNA mixtures [34] |
| Proteinase K | Digests potentially contaminating glycoproteins | High-concentration treatment ensures clean glycoRNA isolation [34] |
| HISAT-StringTie-Ballgown | Bioinformatics pipeline for transcriptome analysis | Identifies and quantifies novel glycoRNA subtypes [33] [35] |
The following diagram illustrates the complete Clier-seq workflow from cell culture to data analysis:
Key Findings from Transcriptome-Wide GlycoRNA Profiling
Application of Clier-seq across multiple cell types, including epithelial cells and B cells, has revealed a previously unappreciated diversity of glycoRNA species and their specific characteristics [33] [35].
Primary RNA Targets of Glycosylation
Clier-seq analysis has demonstrated that glycosylation does not randomly affect RNA molecules but targets specific RNA classes:
- Transfer RNAs (tRNAs): Particularly tRNAs for serine, threonine, valine, and lysine emerge as primary glycosylation targets, suggesting a potential link between translation machinery and cell surface signaling [33] [35].
- Vault RNAs (vtRNAs): Specifically vtRNA2-1 has been identified as glycosylated, connecting these RNAs to extracellular functions beyond their known roles in intracellular ribonucleoprotein complexes [33] [35].
- Long Non-coding RNAs: Several novel glycosylated lncRNAs ranging from 200-400 nucleotides in length have been discovered, significantly expanding the known size distribution of glycoRNAs beyond the previously characterized small RNAs under 200 nt [33] [35].
Size Distribution and Cellular Localization
Comprehensive characterization using Clier-seq has revealed that glycoRNAs are predominantly below 2,000 nucleotides in both epithelial and B cells, with a remarkable concentration in the 50-2,000 nt range that the method was optimized to capture [33] [35]. These molecules are notably enriched on the cell surface, where they potentially interact with various extracellular proteins, including Siglec receptors and other lectins [1] [14].
Table 2: GlycoRNA Species Identified Through Clier-seq Analysis
| GlycoRNA Category | Specific Examples | Length Range | Functional Implications |
|---|---|---|---|
| Transfer RNAs (tRNAs) | tRNAs (Ser, Thr, Val, Lys) | ~70-90 nt | Potential link between translation and cell surface signaling [33] [35] |
| Vault RNAs (vtRNAs) | vtRNA2-1 | ~80-100 nt | Extracellular functions beyond intracellular complex formation [33] [35] |
| Long Non-coding RNAs | Novel lncRNAs | 200-400 nt | Expansion of glycoRNA size distribution and potential functions [33] [35] |
Complementary and Validation Methods
Robust glycoRNA research requires orthogonal methods to validate findings and provide additional layers of biological information.
Biochemical and Imaging Approaches
- Clier-qPCR: Provides orthogonal validation of Clier-seq results through targeted quantification of candidate glycoRNAs, ensuring low false-positive rates [33] [35].
- ARPLA (Aptamer and RNA in situ hybridization-mediated proximity ligation assay): Enables high-sensitivity visualization of glycoRNAs at the single-cell level, revealing that glycoRNAs undergo intracellular trafficking via SNARE protein-mediated secretory exocytosis [1].
- rPAL (RNA Periodate Oxidation and Labeling): Uses mild periodate oxidation of sialic acid diols followed by biotinylation for glycoRNA enrichment without prior metabolic labeling [34] [1].
- Mass Spectrometry: LC-MS/MS and MALDI-TOF approaches characterize attached glycan structures and have helped identify 3-(3-amino-3-carboxypropyl)uridine (acp3U) as a potential nucleotide anchoring site for glycan attachment [1] [36].
Functional Assessment Methods
- Sialidase Digestion: Enzymatic removal of sialic acids confirms the glycosylated nature of identified RNAs and helps validate enrichment specificity [34].
- Extracellular RNase Treatment: Used to dissociate glycoRNA-cell-surface RNA binding protein (csRBP) complexes, demonstrating the functional importance of RNA in maintaining these surface domains [37].
- Siglec Binding Assays: Investigate interactions between glycoRNAs and immunoregulatory receptors, providing insight into potential biological functions [1] [14].
The following diagram illustrates the relationship between glycoRNAs, binding proteins, and their cellular functions:
Biological Significance and Research Applications
The discovery of glycoRNAs and the development of methods like Clier-seq have opened new avenues for understanding cellular biology and disease mechanisms.
Role in Immune Regulation and Cell Signaling
GlycoRNAs interact with members of the Siglec (sialic acid-binding immunoglobulin-like lectin) receptor family, which are known to play critical roles in immune checkpoint regulation and tumor immune evasion [1] [14]. This interaction suggests glycoRNAs may participate in self/non-self discrimination and modulate immune cell activity, positioning them as potential regulators of autoimmune processes and cancer immunity [1] [14].
Cell Surface Organization and Molecular Trafficking
Recent research has revealed that glycoRNAs form specialized domains on the cell surface in coordination with cell-surface RNA-binding proteins (csRBPs) such as nucleolin, hnRNP-U, YBX1, and DDX21 [37]. These csRBP-glycoRNA complexes create a tessellated pattern on the plasma membrane and are functionally important for the uptake of cell-penetrating peptides like TAT, suggesting a previously unrecognized role for RNA in organizing the cell surface and facilitating molecular transport [37].
Implications for Disease and Therapeutics
The homology between cell surface-associated glycoRNAs and disease-associated small RNAs suggests potential roles in pathological processes [1]. Specific applications include:
- Biomarker Discovery: Identification of disease-specific glycoRNA signatures in cancer and potentially autoimmune conditions [34] [36].
- Drug Target Validation: Targeting glycoRNA-biogenesis enzymes or their interactions with immune receptors represents a novel therapeutic strategy [36].
- Mechanistic Studies: Elucidating how glycoRNA expression changes contribute to disease progression, particularly in cancer and neuroinflammatory conditions [36].
Clier-seq represents a transformative methodology that has fundamentally advanced the field of glycoRNA research by enabling comprehensive, transcriptome-wide identification of these elusive biomolecules. By integrating metabolic labeling, click chemistry, and sophisticated bioinformatics, this approach has revealed the surprising diversity of glycosylated RNAs, their specific molecular characteristics, and their potential biological significance. The continued refinement of Clier-seq and complementary methods will undoubtedly accelerate our understanding of how glycoRNAs contribute to normal physiology and disease states, potentially opening new avenues for diagnostic and therapeutic development. As the field matures, standardized pipelines like Clier-seq will be essential for generating reproducible, biologically meaningful data that can bridge the historical divide between RNA biology and glycobiology.
The recent discovery of glycosylated RNA (glycoRNA) has unveiled a new class of biomolecules with significant potential in cancer biology. This technical guide details the translation of a novel detection method—dual-recognition Förster Resonance Energy Transfer (drFRET)—for clinical cancer diagnostics. The drFRET technique enables sensitive, in-situ profiling of glycoRNAs on small extracellular vesicles (sEVs) from minimal biofluid volumes. We provide a comprehensive overview of the core technology, experimental protocols, and analytical frameworks, supported by quantitative clinical performance data from a 100-patient cohort. This document serves as a technical resource for researchers and drug development professionals working at the intersection of glycobiology, RNA science, and liquid biopsy development.
Glycosylated RNAs (glycoRNAs) are an emerging class of biomolecules where RNA is covalently modified with N-glycans, challenging the long-standing paradigm that glycosylation was exclusive to proteins and lipids [1] [24]. These molecules are primarily composed of small non-coding RNAs—including Y RNAs, tRNAs, snRNAs, and snoRNAs—modified with sialic acid-terminated N-glycans [1] [24].
The discovery of glycoRNA has created a new paradigm for understanding cell surface biology and intercellular communication. The primary function of cell surface glycoRNAs appears to be immunomodulation. They serve as ligands for Siglec family receptors (particularly Siglec-10 and Siglec-11) and P-selectin on endothelial cells, positioning them as key regulators of immune cell trafficking, inflammatory responses, and cellular adhesion [1] [8]. Notably, their expression is frequently dysregulated in cancer, creating unique molecular signatures that offer new diagnostic and therapeutic opportunities [8].
The presence of glycoRNAs on small extracellular vesicles (sEVs) is of particular diagnostic interest. sEVs are lipid-bilayer enclosed nanoparticles (30-150 nm) secreted by all cells that play crucial roles in intercellular communication by transferring proteins, nucleic acids, and other biomolecules between cells [38]. Tumor-derived sEVs have been shown to promote cancer progression by facilitating immune suppression, remodeling the tumor microenvironment, and mediating metastatic dissemination [38] [39]. The anchoring of glycoRNAs on sEVs suggests they may reflect concealed biochemical information from parent cells and play functional roles in vesicle adhesion, cellular internalization, and the construction of pre-metastatic niches [21] [40]. This combination of stable vesicular packaging and disease-specific molecular patterns makes sEV glycoRNAs exceptionally promising biomarkers for liquid biopsy applications.
Core Technology: The drFRET Detection Platform
Fundamental Principles
The dual-recognition Förster Resonance Energy Transfer (drFRET) strategy is specifically designed to overcome the limitations of previous glycoRNA detection methods, which often required intricate experimental designs and could not simultaneously provide sequence and spatial information [21]. The core innovation of drFRET lies in its requirement for dual recognition of both the RNA sequence and the glycan moiety to generate a detectable signal.
The platform utilizes two distinct functionalized DNA probes that must simultaneously bind in close spatial proximity (1-10 nm) on a target glycoRNA molecule [21] [40]:
- Glycan Recognition Probe (GRP): A DNA aptamer conjugated with a Cy3 donor fluorescent dye that specifically recognizes and binds to N-acetylneuraminic acid (Neu5Ac), the sialic acid terminus commonly found on glycoRNA N-glycans.
- In Situ Hybridization Probe (ISHP): A complementary oligonucleotide chain labeled with a Cy5 acceptor fluorescent dye that specifically hybridizes to the target RNA sequence through base pairing.
When both probes successfully bind to their respective targets on the same glycoRNA molecule and come within the required 1-10 nm proximity, the excited state of the Cy3 donor fluorophore transfers energy to the Cy5 acceptor via dipole-dipole coupling, generating a sensitized emission FRET signal [21]. This dual-recognition requirement effectively prevents false-positive signals that could arise from single-probe binding events, ensuring high specificity.
Table 1: Key Technical Specifications of the drFRET Platform
| Parameter | Specification | Clinical Significance |
|---|---|---|
| Sample Volume | 10 μL initial biofluid [21] | Enables analysis from finger-prick volumes |
| Target Molecule | Neu5Ac-modified glycoRNAs on sEVs [21] [40] | Specific to sialylated glycoRNA species |
| Recognition Mechanism | Dual probe (glycan + RNA sequence) [21] | Eliminates single-probe false positives |
| Detection Limit | Single-vesicle imaging capability [21] | High sensitivity for rare tumor-derived vesicles |
| Signal Readout | Sensitized emission FRET (Cy3→Cy5) [21] [40] | Spectral cross-interference eliminated via correction |
Workflow Visualization
The following diagram illustrates the core drFRET detection mechanism and experimental workflow:
Experimental Protocols & Methodologies
sEV Isolation and Preparation
The drFRET protocol begins with the isolation of high-purity sEVs from minimal biofluid volumes (10 μL initial volume) using classical differential ultracentrifugation with RNase inhibitors to preserve RNA integrity [21]. Key methodological considerations include:
- Centrifugation Parameters: Sequential centrifugation steps to remove cells, debris, and larger vesicles, with final ultracentrifugation at 100,000-120,000 × g to pellet sEVs [21] [38].
- RNase Inhibition: Inclusion of RNase inhibitors throughout the isolation process to prevent degradation of surface-exposed glycoRNAs.
- Purity Validation: Assessment of sEV preparation using established markers (e.g., CD63, CD81, TSG101) and exclusion of non-vesicular contaminants [38].
drFRET Probe Design and Validation
The design and validation of the dual DNA probes are critical for assay success:
- Glycan Recognition Probe (GRP) Design: Selection of DNA aptamers with high affinity and specificity for Neu5Ac through Systematic Evolution of Ligands by Exponential Enrichment (SELEX) or utilization of previously characterized aptamers [21] [40]. The GRP is conjugated with Cy3 donor fluorophore at specific positions that do not interfere with target binding.
- In Situ Hybridization Probe (ISHP) Design: Design of complementary oligonucleotides (typically 18-25 nucleotides) targeting conserved regions of prevalent glycoRNA species (e.g., Y RNAs, specific tRNAs). The ISHP is labeled with Cy5 acceptor fluorophore at terminal or internal positions with minimal impact on hybridization efficiency.
- Probe Validation: Specificity validation through control experiments including:
- Competition assays with free Neu5Ac (for GRP)
- Hybridization with non-target RNAs (for ISHP)
- Single-probe control experiments to confirm absence of FRET signal
FRET Imaging and Signal Acquisition
The imaging protocol involves precise instrumentation setup and signal processing:
- Microscopy Platform: Confocal or super-resolution microscopy systems capable of sensitive fluorescence detection at single-vesicle level [21].
- Excitation/Detection Parameters: Cy3 excitation at 550 nm, with simultaneous detection of donor emission (565-595 nm) and sensitized acceptor emission (665-695 nm) [21] [40].
- Spectral Unmixing: Implementation of spectral correction algorithms to eliminate cross-talk and direct excitation contributions, ensuring FRET signals originate exclusively from true energy transfer events [21] [40].
- Image Analysis: Automated vesicle identification and FRET efficiency calculation using custom or commercial software packages, with FRET efficiency (E) calculated as E = IA/(ID + IA), where IA is acceptor emission and I_D is donor emission.
Clinical Validation and Diagnostic Performance
Cohort Design and Cancer Typing
The diagnostic performance of the drFRET platform was evaluated in a comprehensive clinical study analyzing sEVs from a 100-patient cohort encompassing six cancer types and non-cancer controls [21] [40]. The study identified five prevalent sEV glycoRNAs that served as the diagnostic signature.
Table 2: Diagnostic Performance of drFRET for sEV GlycoRNA Profiling
| Performance Metric | Result | Study Parameters |
|---|---|---|
| Cancer vs. Non-Cancer | 100% accuracy (95% CI) [21] [40] | 100-patient cohort; 6 cancer types + controls |
| Specific Cancer Typing | 89% overall accuracy (95% CI) [21] [40] | Classification across 6 different cancer types |
| Analytical Sensitivity | Detection from 10 μL biofluid [21] | Minimal sample volume requirement |
| Targets Identified | 5 prevalent sEV glycoRNAs [21] | Derived from 7 cancer cell lines |
Data Analysis and Pattern Recognition
The analysis of drFRET data employs sophisticated computational approaches to extract diagnostic information:
- Dimensionality Reduction: Application of unsupervised hierarchical clustering analysis to the five glycoRNA signals, resulting in completely separated clusters between cancer and non-cancer samples [21] [40].
- Classification Modeling: Construction of a precise cancer typing model through principal coordinate analysis, achieving the 89% accuracy in classifying specific cancer types across the six cancer types evaluated [21].
- Signal Integration: Utilization of the unweighted sum of the five individual glycoRNA levels as a highly effective diagnostic classifier, suggesting these markers may function as a coordinated signaling system [21].
The Scientist's Toolkit: Essential Research Reagents
Successful implementation of drFRET for glycoRNA detection requires specific reagents and materials. The following table details key solutions and their functional roles in the experimental workflow.
Table 3: Essential Research Reagents for drFRET-based GlycoRNA Detection
| Reagent Category | Specific Examples | Function & Application |
|---|---|---|
| Glycan Recognition Probes | Neu5Ac-specific DNA aptamer-Cy3 conjugates [21] [40] | Specific recognition of sialic acid termini on glycoRNA glycans; FRET donor |
| RNA Hybridization Probes | Sequence-specific DNA oligonucleotides-Cy5 conjugates [21] [40] | Complementary binding to target glycoRNA sequences; FRET acceptor |
| sEV Isolation Reagents | Ultracentrifugation buffers; RNase inhibitors; Size-exclusion columns [21] [38] | Purification of intact sEVs with preserved surface glycoRNAs |
| Fluorescence Detection | Confocal microscopy systems; Spectral unmixing software [21] | Sensitive detection of FRET signals at single-vesicle resolution |
| Analytical Tools | Dimensionality reduction algorithms; Clustering software [21] | Pattern recognition and classification of glycoRNA signatures |
| Desmethyl Celecoxib | Desmethyl Celecoxib, CAS:170569-87-6, MF:C16H12F3N3O2S, MW:367.3 g/mol | Chemical Reagent |
Biological Significance and Functional Insights
Beyond its diagnostic utility, the drFRET platform has yielded fundamental insights into glycoRNA biology and function. The technology has revealed that sEV glycoRNAs specifically interact with Siglec proteins (particularly Siglec-10 and Siglec-11) and P-selectin, interactions that are critical for sEV cellular internalization [21] [8]. These findings position glycoRNAs as active participants in cell-cell communication rather than merely passive biomarkers.
The following diagram illustrates the current understanding of glycoRNA biosynthesis and function based on findings enabled by drFRET and related technologies:
The diagram summarizes key aspects of glycoRNA biology: their biosynthesis begins with acp³U modification of RNA during tRNA maturation, followed by trafficking through the secretory pathway where classical N-glycosylation machinery (OST complex) attaches sialylated N-glycans [1] [24]. After packaging into sEVs, surface-displayed glycoRNAs engage in functional interactions with Siglec receptors and P-selectin, mediating critical processes in sEV cellular internalization and inflammatory recruitment [21] [8].
The drFRET platform represents a significant advancement in the toolbox for glycoRNA detection and functional analysis, offering unprecedented sensitivity and specificity for profiling these emerging biomarkers on sEVs. The technology's demonstrated performance in cancer diagnosis—achieving 100% accuracy in distinguishing cancers from non-cancer controls and 89% accuracy in classifying specific cancer types—highlights the clinical potential of sEV glycoRNAs as biomarkers [21] [40].
For researchers and drug development professionals, drFRET offers a versatile platform for both diagnostic applications and fundamental investigations into glycoRNA biology. Future developments will likely focus on expanding the panel of detectable glycoRNA species, automating the analytical workflow for higher throughput, and validating the technology across larger, multi-center clinical cohorts. As our understanding of glycoRNA biosynthesis and function continues to evolve, drFRET stands positioned as a key enabling technology for translating this novel class of biomolecules into clinical practice.
Navigating Challenges: Technical Hurdles and Functional Insights in GlycoRNA Research
The recent discovery of glycoRNA—small non-coding RNAs modified with sialylated glycans—has fundamentally challenged long-standing biological principles. This fresh component of the cell surface glycome is implicated in immune recognition and cell communication. However, its existence presents a central biosynthetic paradox: the established machinery for N-glycosylation resides within the endoplasmic reticulum (ER) and Golgi apparatus, compartments from which RNA is conventionally excluded. This whitepaper synthesizes current research to dissect the leading hypotheses and experimental evidence that may resolve this puzzle, focusing on the potential pathways enabling RNA to access the glycosylation machinery. We further provide a detailed toolkit for researchers, including standardized protocols, key reagents, and visual frameworks to accelerate investigation into this nascent field.
GlycoRNAs are a novel class of biomolecules where small non-coding RNAs (e.g., Y RNAs, snRNAs, snoRNAs) are covalently modified with N-glycans rich in sialic acid and fucose [6] [1]. Discovered in 2021, these molecules are predominantly displayed on the extracellular surface of cells, where they function as ligands for immune receptors such as Siglecs (sialic acid-binding immunoglobulin-like lectins) [6] [8]. Their discovery immediately raised a fundamental question in cellular biology: How are RNAs glycosylated?
The canonical N-glycosylation pathway for proteins is well-defined. It involves the assembly of a lipid-linked oligosaccharide (LLO) precursor in the ER membrane, followed by the enzymatic transfer of the glycan to asparagine residues within a specific sequon (N-X-S/T) on a nascent protein. This process is catalyzed by the oligosaccharyltransferase (OST) complex [41] [42]. Subsequent glycan maturation occurs in the Golgi apparatus. Critically, the entire process is compartmentalized within the ER-Golgi network, and RNA is not known to traffic through these spaces [6]. This creates the central glycoRNA paradox: the machinery and the substrate appear to exist in separate cellular realms. This whitepaper explores the leading theories and experimental data aimed at resolving this mystery.
Competing Hypotheses for GlycoRNA Biogenesis
Several non-mutually exclusive models have been proposed to explain the biogenesis of glycoRNA. The table below summarizes the core principles, supporting evidence, and unresolved questions for each.
Table 1: Leading Hypotheses for GlycoRNA Biosynthesis
| Hypothesis | Core Principle | Supporting Evidence | Unresolved Questions |
|---|---|---|---|
| Direct RNA Glycosylation in the ER/Golgi | RNA is actively transported into the ER or Golgi lumen to be glycosylated by the canonical machinery. | - GlycoRNA formation is impaired by genetic knockout of LLO biosynthesis enzymes and OST inhibition [6] [1]. | - What is the mechanism for RNA import into the ER/Golgi?- Is there a specific RNA transporter? |
| Protein-Mediated Anchoring | A glycoprotein acts as a linker, binding both an RNA and a glycan, creating a ternary complex that is mistaken for a direct conjugate. | - Some glycan signals in "glycoRNA" preps are resistant to RNase but sensitive to Proteinase K, suggesting glycoprotein contamination [9].- LAMP1 glycoprotein co-purifies with small RNA [9]. | - Is this the sole explanation, or one of several pathways?- Can this model explain all glycoRNA observations? |
| External Surface Assembly | Glycan precursors are exported to the cell surface where glycosyltransferases modify surface-bound RNAs. | - The presence of various cell-surface RNA-binding proteins (csRBPs) that form nanoclusters with glycoRNAs [1]. | - Is the full N-glycan biosynthetic pathway available at the plasma membrane?- What is the energy source for this process? |
The following diagram illustrates the logical flow of these competing hypotheses and their connection to experimental observations.
Key Enzymatic Machinery and Chemical Linkage
The Role of the Oligosaccharyltransferase (OST) Complex
A critical finding is that glycoRNA biogenesis is dependent on the canonical N-glycan biosynthetic pathway. Genetic or pharmacological inhibition of the OST complex, the enzyme responsible for transferring the pre-assembled glycan from the LLO to an acceptor substrate, significantly diminishes glycoRNA production [6]. This provides strong evidence that the formation of glycoRNAs is not a peripheral or non-specific event but is directly tied to the core enzymatic machinery of N-linked glycosylation. This dependency is a cornerstone of the biogenesis puzzle.
The acp3U Linkage
A major breakthrough in understanding the chemical basis of glycoRNA was the identification of a potential glycosylation site on the RNA itself. Using RNA-optimized periodate oxidation and aldehyde ligation (rPAL) coupled with mass spectrometry, researchers proposed that the modified RNA base 3-(3-amino-3-carboxypropyl)uridine (acp3U) serves as the primary attachment site for N-glycans [1] [25]. acp3U is a highly conserved modified uridine found in bacterial and mammalian tRNAs, known to enhance RNA thermostability [1]. This discovery provides a plausible chemical bridge, suggesting the glycosidic linkage may be similar, though not identical, to the asparagine-N-acetylglucosamine bond in glycoproteins.
Table 2: Key Enzymes and Molecules in GlycoRNA Biogenesis
| Molecule/Enzyme | Type | Function in GlycoRNA Biogenesis | Experimental Manipulation |
|---|---|---|---|
| Oligosaccharyltransferase (OST) | Enzyme Complex | Catalyzes the transfer of glycan from LLO to acceptor substrate; essential for glycoRNA production. | Inhibited genetically (CRISPR) or pharmacologically to diminish glycoRNA yield [6]. |
| acp3U (3-(3-amino-3-carboxypropyl)uridine) | Modified Nucleotide | Proposed covalent attachment site for N-glycans on the RNA molecule [1] [25]. | Detected and characterized via rPAL and SWATH-MS [1]. |
| alg Genes (e.g., ALG1, ALG2) | Glycosyltransferases | Involved in the step-wise biosynthesis of the LLO donor substrate in the ER [42]. | Knockdown/knockout expected to impair glycoRNA production by limiting LLO substrate. |
| Siglec Receptors (e.g., Siglec-11, -14) | Lectin Receptors | Bind to sialylated glycans on cell-surface glycoRNAs, mediating immune cell interactions [6] [8]. | Used as soluble Fc reagents in flow cytometry to validate glycoRNA binding [6]. |
Essential Research Protocols and Reagents
To investigate the biosynthetic pathway of glycoRNA, researchers have developed and adapted a suite of biochemical and cell biological methods. The workflow below outlines a standard pipeline for metabolic labeling, purification, and validation of glycoRNAs, highlighting key decision points.
The Scientist's Toolkit: Key Research Reagents
Table 3: Essential Reagents for GlycoRNA Research
| Research Reagent | Function/Application | Key Consideration |
|---|---|---|
| Ac4ManNAz (Peracetylated N-azidoacetylmannosamine) | Metabolic precursor for azide-modified sialic acid. Enables bioorthogonal labeling of nascent glycans on RNA [6] [9]. | The azido group allows for subsequent click chemistry conjugation. |
| DBCO-Biotin (Dibenzocyclooctyne-Biotin) | Click chemistry reagent. The DBCO group reacts with the azide on Ac4ManNAz-labeled glycans, while biotin enables streptavidin-based purification/detection [6]. | Strain-promoted azide-alkyne cycloaddition (SPAAC) is copper-free, preserving RNA integrity. |
| PNGase F | Enzyme that cleaves between the innermost GlcNAc and asparagine in N-glycans. Used to probe the nature of the glycan-RNA linkage [6] [25]. | GlycoRNA sensitivity to PNGase F suggests a similar amide-based linkage, implicating acp3U. |
| Proteinase K (in Denaturing Buffer) | Protease used under denaturing conditions (e.g., with SDS) to rigorously eliminate glycoprotein contamination from RNA preps [9]. | A critical control to distinguish true glycoRNA from protein-RNA-glycan complexes. |
| Silica Spin Columns (e.g., Zymo Research) | For size-based separation of small RNAs (<200 nt) and desalting after click chemistry reactions [6] [9]. | Purification post-RNase treatment can remove glycosylated molecules, impacting results [9]. |
| Anti-Siglec-Fc Chimeras | Soluble recombinant proteins used to validate the functional binding of cell-surface glycoRNAs to Siglec receptors via flow cytometry [6]. | Confirms the biological relevance of glycoRNAs as immune ligands. |
Critical Analysis and Contradictory Evidence
The field of glycoRNA biosynthesis is dynamically evolving, and recent findings call for a careful re-evaluation of initial results. A pivotal study demonstrated that the robustness of the glycoRNA signal is highly dependent on specific purification steps [9]. This research found that:
- Glycosylated molecules isolated using the standard protocol showed resistance to RNase A/T1 but were sensitive to proteinase K digestion under denaturing conditions.
- Mass spectrometry analysis confirmed that glycoproteins (e.g., LAMP1) co-purify with small RNA preparations.
- The binding of these glycosylated molecules to the purification silica columns is impaired after RNase treatment, suggesting that the RNA may act as a scaffold that facilitates the co-purification of glycoproteins, which are the actual source of the glycan signal in some experiments [9].
These findings do not necessarily disprove the existence of glycoRNA but highlight that glycoprotein contamination is a considerable source of artifacts using current protocols. Future research must incorporate stringent denaturing protease controls and develop novel purification strategies to unequivocally isolate bona fide glycoRNA molecules.
The quest to resolve the biosynthetic puzzle of glycoRNA is at a fascinating juncture. Strong evidence, including OST-dependence and the identification of acp3U, points toward a novel, direct RNA modification. Yet, equally compelling data warns of significant experimental pitfalls from co-purifying glycoproteins. The path forward requires a concerted effort to:
- Develop Novel Purification Strategies: Create methods that can definitively separate covalently linked glycoRNA from any non-covalently associated glycoprotein complexes.
- Elucidate the Transport Mechanism: Identify the potential transporters or pathways that could bring RNA into proximity with the glycosylation machinery, or confirm the existence of ecto-glycosyltransferases.
- Validate the acp3U Linkage In Vivo: Confirm that acp3U is the physiological attachment site and delineate the enzymes responsible for creating this linkage.
Unraveling this puzzle will not only satisfy a fundamental question in cell biology but also open new avenues for therapeutic intervention. Given the role of cell-surface glycoRNAs as Siglec ligands in immune regulation and cancer, understanding their biogenesis could reveal new targets for immunotherapy and other treatment modalities [1] [8]. The coming years promise to be critical in solidifying the mechanisms behind this groundbreaking discovery.
Glycosylated RNA, or glycoRNA, represents a groundbreaking discovery in molecular biology, challenging the long-held paradigm that glycosylation modifications are exclusive to proteins and lipids. These molecules, consisting of small non-coding RNAs covalently linked to sialic acid-rich N-glycans, have been found on the cell surface where they participate in critical biological processes, particularly immune recognition and cell-to-cell communication [1] [43]. GlycoRNAs interact with immunoregulatory receptors such as Siglecs (sialic acid-binding immunoglobulin-like lectins), positioning them as potential key regulators in immune homeostasis, autoimmune diseases, and cancer progression [1] [14] [43].
The detection of these molecules presents significant technical challenges due to their low abundance and the complex nature of their hybrid RNA-glycan structure. Early skepticism about their existence underscores the critical need for highly sensitive and specific detection methodologies [9] [24]. This technical guide examines current and emerging strategies to overcome these sensitivity limitations, providing researchers with actionable methodologies for advancing this rapidly evolving field.
Technical Challenges in GlycoRNA Detection
The reliable detection of glycoRNAs is hampered by several inherent technical obstacles that must be addressed through methodological optimization:
- Low Abundance: GlycoRNAs constitute a minute fraction of the total cellular RNA pool, necessitating enrichment strategies prior to detection [1] [24].
- Hybrid Nature: Their unique composition requires simultaneous consideration of both RNA and glycan properties, complicating standard analytical approaches [44].
- Contamination Risks: Glycoproteins can co-purify with RNA preparations, leading to potential false positives if not properly controlled [9].
- Structural Complexity: The precise molecular linkage between glycans and RNA involves the modified nucleotide acp3U (3-(3-amino-3-carboxypropyl)uridine), requiring specialized methods for characterization [1] [24].
Recent studies have highlighted the importance of rigorous controls, with evidence suggesting that some glycans detected in RNA preparations may originate from contaminating glycoproteins that resist standard proteinase K treatment under nondenaturing conditions [9]. This underscores the critical need for denaturing conditions during enzymatic treatments and implementation of multiple orthogonal detection strategies.
Advanced Detection Strategies
Metabolic Labeling and Chemical Tagging
Metabolic labeling leverages the cell's own biosynthetic machinery to incorporate tagged sugars into glycans, providing a highly specific handle for glycoRNA detection.
Experimental Protocol:
- Cell Culture and Labeling: Culture cells in medium supplemented with 100 µM Ac4ManNAz, 100 µM GalNAc, and 10 µM D-galactose for 40 hours [9].
- RNA Extraction: Lyse cells in TRIzol reagent, incubate at 37°C for 10 minutes to ensure complete lysis.
- Phase Separation: Add 0.2 volumes chloroform, centrifuge at 4,000g for 10 minutes.
- RNA Precipitation: Mix aqueous phase with 1.1 volumes isopropanol, precipitate at -20°C for 1 hour.
- Silica Column Purification: Use Zymo Spin IC columns (for ≤70 µg RNA) or IIICG columns (for ≤350 µg RNA) with three wash steps [9].
- Click Chemistry Reaction: Incubate with fluorescent azide probes (e.g., DBCO-fluorophore) via strain-promoted azide-alkyne cycloaddition.
- Detection: Analyze via northwestern blotting with appropriate controls [24].
RNA-Optimized Periodate Oxidation and Aldehyde Ligation (rPAL)
The rPAL method exploits the chemical properties of glycan diols for highly sensitive detection, providing approximately 25-fold increased sensitivity over metabolic labeling approaches [1] [24].
Experimental Protocol:
- Sample Preparation: Isolate native glycoRNAs using silica column purification under RNase-free conditions.
- Oxidation Reaction: Treat samples with periodate (2-5 mM final concentration) to oxidize vicinal diols in sialic acid residues.
- Aldehyde Capture: Incubate with aminooxy-functionalized solid-phase supports to form stable oxime bonds.
- Washing: Stringently wash to remove non-specifically bound material.
- Elution and Analysis: Elute bound glycoRNAs for downstream northern blot analysis or mass spectrometry.
- Mass Spectrometry Characterization: Utilize high-sensitivity MS to identify acp3U as the key nucleotide anchoring site for glycan attachment [1].
Dual-Recognition FRET (drFRET) for Extracellular Vesicles
drFRET enables ultrasensitive detection of glycoRNAs in biofluids by requiring simultaneous recognition of both RNA and glycan components, significantly reducing background signal.
Experimental Protocol:
- Vesicle Isolation: Purify small extracellular vesicles (sEVs) from cell culture supernatants or clinical serum samples via ultracentrifugation or commercial kits.
- Probe Design: Design two complementary probes:
- Glycan-recognition probe: Lectin (e.g., WGA) conjugated to FRET donor
- RNA-recognition probe: Sequence-specific oligonucleotide conjugated to FRET acceptor
- Incubation: Mix probes with sEV samples and incubate to allow simultaneous binding.
- FRET Measurement: Excite donor fluorophore and measure energy transfer to acceptor, indicating proximity (<10 nm) of binding sites.
- Quantification: Use standard curves to quantify glycoRNA levels in samples.
- Clinical Application: This method has demonstrated 100% accuracy in distinguishing cancer versus control samples and approximately 90% accuracy in cancer subclassification within patient cohorts [24].
Aptamer and RNA In Situ Hybridization-Mediated Proximity Ligation Assay (ARPLA)
ARPLA provides single-cell resolution for glycoRNA visualization by combining dual recognition with enzymatic signal amplification.
Experimental Protocol:
- Cell Preparation: Fix cells under mild conditions to preserve epitopes and RNA integrity.
- Dual Recognition:
- Apply glycan-specific aptamer or lectin
- Apply RNA-specific oligonucleotide probe
- Proximity Ligation: When both probes bind in close proximity, trigger an in situ ligation reaction.
- Signal Amplification: Perform rolling circle amplification of complementary DNA.
- Detection: Use fluorescently labeled oligonucleotides for signal output and visualize via confocal microscopy.
- Trafficking Studies: This approach has revealed that glycoRNAs undergo intracellular trafficking via SNARE protein-mediated secretory exocytosis [1].
Comparative Analysis of Detection Methods
Table 1: Performance Comparison of GlycoRNA Detection Methods
| Method | Sensitivity | Spatial Resolution | Throughput | Key Applications | Limitations |
|---|---|---|---|---|---|
| Metabolic Labeling + Northwestern Blot | Moderate (µg RNA input) | Bulk population | Low | Initial discovery, validation | Requires unnatural sugars, potential metabolic effects |
| rPAL | High (~25x metabolic) | Bulk population | Medium | GlycoRNA characterization, linkage identification | Chemical oxidation may damage samples |
| drFRET | Very High (10 µL biofluid) | Single vesicle | High | Clinical diagnostics, biofluid analysis | Requires specialized instrumentation |
| ARPLA | Single-molecule | Single-cell | Medium | Subcellular localization, trafficking studies | Complex workflow, optimization intensive |
| Lectin Proximity Labeling | Moderate | Subcellular | Medium | Interactome studies, spatial organization | Potential off-target lectin binding |
Table 2: Essential Research Reagents for GlycoRNA Studies
| Reagent Category | Specific Examples | Function | Considerations |
|---|---|---|---|
| Metabolic Labels | Ac4ManNAz, Ac4GalNAz | Incorporates click-compatible handles into glycans | Concentration optimization required to avoid toxicity |
| Click Chemistry Reagents | DBCO-fluorophore, Azide-biotin | Covalent tagging for detection and enrichment | Strain-promoted cycloadditions preferred for bioorthogonality |
| Enrichment Matrices | Aminooxy-functionalized beads, Streptavidin resins | Isolation of low-abundance glycoRNAs | Nonspecific binding must be controlled |
| Lectins | WGA (Wheat Germ Agglutinin), SNA (Sambucus Nigra Lectin) | Glycan recognition and purification | Specificity varies; lectin panels recommended |
| Nucleases | RNase A/T1 cocktail, Benzonase | Specificity controls and sample cleanup | Requires careful titration and validation |
| Proteases | Proteinase K (with/without denaturants) | Contamination control | Denaturing conditions essential for complete digestion [9] |
Experimental Design Considerations
Contamination Control and Validation
The potential for glycoprotein contamination in glycoRNA preparations represents a significant challenge that must be rigorously addressed:
- Proteinase K Treatment Optimization: Standard proteinase K digestion under nondenaturing conditions may be insufficient to eliminate all contaminating glycoproteins. Implement denaturing conditions using buffers containing SDS and 2-mercaptoethanol to ensure complete protein degradation [9].
- Orthogonal Validation: Always employ multiple detection methods with different principles (e.g., metabolic labeling + rPAL) to confirm results.
- RNase Sensitivity Controls: Include rigorous RNase A/T1 treatments (≥30 minutes at 37°C) to confirm the RNA-dependent nature of signals.
- Mass Spectrometry Verification: Utilize high-sensitivity MS to directly identify the acp3U-glycan linkage and distinguish true glycoRNAs from contaminants [1].
Sensitivity Enhancement Strategies
- Pre-enrichment Methods: Combine multiple enrichment strategies (e.g., lectin pull-down followed by click chemistry enrichment).
- Signal Amplification: Implement enzymatic amplification (e.g., rolling circle amplification) for low-abundance targets.
- Background Reduction: Use dual-recognition strategies (e.g., drFRET, ARPLA) to significantly lower false positive rates.
- Workflow Integration: Couple detection methods with small RNA sequencing (NEXTFLEX Small RNA Sequencing Kit V4) to simultaneously identify RNA sequences and glycosylation status [24].
Future Directions and Concluding Remarks
The field of glycoRNA research is rapidly evolving, with several promising technological developments on the horizon:
- Machine Learning Integration: AI-assisted analysis of glycan mass spectrometry data and prediction of glycosylation sites [45].
- Automated Platforms: Robotic fluidic systems for reproducible glycoRNA enrichment and analysis [45].
- Single-Cell Multi-omics: Combining glycoRNA detection with transcriptomic and proteomic profiling at single-cell resolution.
- Engineered Enzymes: Rationally designed enzymes for specific glycoRNA labeling and manipulation [45].
As detection methods continue to improve in sensitivity and specificity, researchers will be better positioned to unravel the functional significance of these novel molecules in immune regulation, cancer biology, and cell-cell communication. The strategies outlined in this technical guide provide a foundation for advancing these efforts, enabling more robust investigation of glycoRNAs despite their challenging low-abundance nature.
The remarkable progress in this field since its initial discovery in 2021 demonstrates how methodological innovations can open entirely new areas of biological inquiry, reminding us that technical limitations often conceal biological realities waiting to be uncovered.
The study of the cell surface has long been dominated by the paradigm that glycoproteins and glycolipids serve as the primary mediators of intercellular communication and immune recognition. The recent discovery of glycosylated RNA (glycoRNA) has fundamentally challenged this perspective, revealing an entirely new class of biomolecules that participate in immune regulation [1]. GlycoRNAs are defined as small non-coding RNAs modified with sialylated and fucosylated N-glycans, and they are present on the cell surface where they can interact with various immune receptors [1] [6] [25]. This unexpected finding bridges the previously distinct fields of RNA biology and glycobiology, suggesting novel mechanisms through which cells may regulate immune responses.
The significance of glycoRNA extends beyond its mere existence to its potential functional roles in health and disease. These molecules appear to be ligands for sialic acid-binding immunoglobulin-like lectins (Siglecs), a family of immunoregulatory receptors [1] [6]. This interaction positions glycoRNAs as potential modulators of immune cell function, with implications for cancer, autoimmune disorders, transplant medicine, and infectious diseases [46] [47] [48]. This whitepaper synthesizes current knowledge on glycoRNA-Siglec interactions, their functional consequences in immune regulation, experimental approaches for their study, and their translational potential in therapeutic development.
Structural and Molecular Characteristics of GlycoRNA
Composition and Cellular Localization
GlycoRNAs constitute a unique class of biomolecules characterized by their dual-component nature:
RNA Components: GlycoRNAs are primarily derived from small non-coding RNA species, including small nuclear RNAs (snRNAs), small nucleolar RNAs (snoRNAs), transfer RNAs (tRNAs), and Y RNAs [1] [6] [25]. Research in glioma cells identified U2 and U4 snRNAs as particularly abundant glycoRNA species [2].
Glycan Structures: The glycans associated with RNA are predominantly N-glycans rich in sialic acid and fucose components [1] [25]. Mass spectrometry analysis has revealed that these glycans consist of complex, sialylated, and fucosylated structures [2].
Cellular Distribution: Contrary to traditional RNA localization patterns, glycoRNAs are predominantly displayed on the extracellular surface of cells [1] [6] [25]. This surface localization is evidenced by their sensitivity to extracellular sialidases and has been visualized using specialized imaging techniques [6] [25].
Biosynthetic Pathway
The mechanism underlying RNA glycosylation remains an active area of investigation, but current evidence points to a biosynthetic process that shares machinery with protein N-glycosylation:
Dependence on Canonical Glycosylation Machinery: GlycoRNA biogenesis requires functional oligosaccharyltransferase (OST) complexes, as demonstrated by diminished glycoRNA production following OST inhibition [1] [6]. This suggests that the process occurs, at least partially, within the endoplasmic reticulum-Golgi pathway.
The Localization Paradox: A fundamental question persists regarding how RNA, typically excluded from the ER-Golgi compartments, accesses the glycosylation machinery. Potential explanations include specialized transport mechanisms or the existence of alternative glycosylation pathways [6].
Glycan-RNA Linkage: The chemical linkage connecting glycans to RNA involves the modified RNA base 3-(3-amino-3-carboxypropyl)uridine (acp3U) [1] [25]. This covalent linkage is stable under denaturing conditions and sensitive to PNGase F treatment, similar to protein N-glycans [25].
Table 1: Key Characteristics of GlycoRNA Molecules
| Feature | Description | Significance |
|---|---|---|
| RNA Type | Small non-coding RNAs (snRNAs, snoRNAs, Y RNAs) | Suggests regulatory rather than coding function [1] [2] |
| Glycan Type | Complex N-glycans with sialic acid and fucose | Similar to glycoprotein glycans; recognized by Siglec receptors [1] [25] |
| Cellular Location | Cell surface, extracellular | Positions glycoRNAs to interact with immune receptors [6] [25] |
| Biosynthesis | OST-dependent, ER-Golgi pathway | Challenges conventional RNA localization paradigms [1] [6] |
| Chemical Linkage | via acp3U modified base | Covalent, stable linkage distinct from protein glycosylation [1] [25] |
Siglec Family: Key Receptors for GlycoRNA Recognition
Siglec Biology and Function
Siglecs (Sialic acid-binding Immunoglobulin-like Lectins) are a family of transmembrane receptors primarily expressed on immune cells that recognize sialic acid-containing glycans [47] [49]. These receptors play crucial roles in immune regulation by distinguishing self from non-self and modulating immune cell activation:
Structural Features: Siglecs contain an extracellular V-set domain that mediates sialic acid binding, followed by varying numbers of C2-set domains, a transmembrane region, and cytoplasmic tails that often contain immunoreceptor tyrosine-based inhibitory motifs (ITIMs) or ITIM-like domains [50] [49].
Immunoregulatory Roles: Most Siglecs function as inhibitory receptors that dampen immune responses when engaged, thereby preventing excessive activation and maintaining immune homeostasis [47] [49]. For instance, Siglec-7 on NK cells and Siglec-9 on myeloid cells deliver inhibitory signals that constrain cytotoxic activity and inflammatory responses [50] [48].
Expression Patterns: Different Siglec family members exhibit restricted expression on specific immune cell subsets. Siglec-7 is notably expressed on natural killer (NK) cells, monocytes, and T lymphocytes, while Siglec-9 is found predominantly on neutrophils and monocytes [50] [47].
GlycoRNA-Siglec Interactions
The discovery that glycoRNAs serve as ligands for Siglec receptors has expanded our understanding of how these immunoregulatory pathways operate:
Specific Binding Partnerships: Research has demonstrated that cell surface glycoRNAs can bind specifically to certain Siglec family members. Studies using Siglec-Fc recombinant proteins revealed that Siglec-11 and Siglec-14 show RNase-sensitive binding to cells [6]. Additional evidence suggests up to 9 of 12 human Siglecs may recognize glycoRNA ligands [1].
Functional Consequences: GlycoRNA-Siglec interactions contribute to immune modulation by engaging inhibitory or activating Siglec pathways. In cancer, these interactions can promote immune evasion, while in transplant medicine, they may help regulate inflammatory responses to transplanted organs [50] [48].
Pathological Significance: Aberrant glycoRNA-Siglec interactions have been implicated in disease processes. In breast cancer, elevated Siglec-7 expression correlates with adverse clinicopathological features and immune suppression [50]. Similarly, in glioma, glycoRNAs contribute to tumor cell proliferation [2].
Figure 1: GlycoRNA-Siglec Immune Regulatory Pathway. This diagram illustrates the proposed mechanism by which cell surface glycoRNAs engage with Siglec receptors on immune cells, leading to intracellular signaling events that modulate immune function.
Methodologies for GlycoRNA Research
Detection and Visualization Techniques
The study of glycoRNAs requires specialized methodologies due to their unique properties and low abundance:
Metabolic Labeling with Ac4ManNAz: Peracetylated N-azidoacetylmannosamine (Ac4ManNAz) serves as a metabolic precursor that incorporates azide-modified sialic acids into glycans, allowing subsequent conjugation with DBCO-biotin via click chemistry for detection and purification [2]. This approach enabled the initial discovery of glycoRNAs and remains a cornerstone technique.
RNA-optimized Periodate Oxidation and Aldehyde Ligation (rPAL): This sensitive method exploits the 1,2-diols in sialic acids for periodate oxidation, generating aldehydes that form stable oxime bonds with aminooxy-functionalized solid supports, enabling specific enrichment of sialylated glycoRNAs [1].
Dual-recognition FRET (drFRET): An advanced imaging technology that enables visualization of glycosylated RNAs in small extracellular vesicles from cancer cell lines and clinical samples. This technique has been used to elucidate interactions between glycosylated RNAs and Siglec-10, Siglec-11, and P-selectin [1].
Aptamer and RNA in situ Hybridization-mediated Proximity Ligation Assay (ARPLA): This method allows high-sensitivity and high-selectivity visualization of glycoRNAs at the single-cell level through dual recognition of glycans and RNA, triggering an in situ ligation reaction followed by rolling circle amplification [1].
Analytical and Functional Characterization Methods
Sequencing Approaches: Small RNA deep sequencing of purified glycoRNAs identifies the specific RNA species that undergo glycosylation. This has revealed enrichment of particular small non-coding RNAs like U2, U4, and Y5 in different cellular contexts [2].
Glycan Analysis: Liquid chromatography-mass spectrometry (LC-MS) characterizes the glycan components associated with RNA, identifying specific modifications such as fucosylation and sialylation patterns [2].
Functional Assays: Standard cell biological assays including CCK-8 for viability, Ki67 staining for proliferation, TUNEL for apoptosis, and adhesion assays evaluate the functional consequences of glycoRNA manipulation [2].
Table 2: Experimental Approaches for GlycoRNA Study
| Method Category | Specific Techniques | Applications | Key Insights Generated |
|---|---|---|---|
| Detection & Enrichment | Ac4ManNAz labeling, rPAL, SPCgRNA | Isolation and identification of glycoRNAs | Demonstrated existence of RNA-glycan conjugates [1] [2] |
| Visualization | drFRET, ARPLA, Northern blot | Spatial localization of glycoRNAs | Confirmed cell surface display and intracellular trafficking [1] |
| Composition Analysis | RNA sequencing, LC-MS, MERR HRP-seq | Characterization of RNA and glycan components | Identified predominant RNA species and glycan structures [1] [2] |
| Functional Analysis | CCK-8, Ki67, TUNEL, adhesion assays | Assessment of biological roles | Revealed roles in cell proliferation and immune function [2] |
| Interaction Studies | Siglec-Fc binding, RNase sensitivity | Mapping receptor-ligand relationships | Established glycoRNAs as Siglec ligands [1] [6] |
Quantitative Data on GlycoRNA in Disease Contexts
GlycoRNA in Cancer
Research across multiple cancer types has revealed quantitative relationships between glycoRNA expression and disease parameters:
Glioma: In U87 and LN229 glioma cell lines, glycoRNAs are highly abundant, predominantly comprising small RNAs. Functional studies demonstrated that depletion of cell-surface glycoRNAs significantly inhibited glioma cell viability and proliferation without affecting adhesion or apoptosis levels at the observed time points [2].
Breast Cancer: Siglec-7 transcripts are significantly upregulated in breast tumor tissues compared to matched adjacent non-invaded tissues. Higher Siglec-7 expression correlates with negative estrogen receptor (ER) and progesterone receptor (PR) status, advanced tumor grades, and unfavorable patient prognosis [50].
Therapeutic Implications: In breast cancer, high Siglec-7 expression associates with increased infiltration of immunosuppressive cells and T-cells with an exhausted phenotype. This expression pattern also predicts resistance to conventional therapies including chemotherapy, endocrine treatments, and immunotherapy [50].
GlycoRNA in Transplant Medicine
Recent evidence highlights the significance of the Siglec-glycoRNA axis in transplant outcomes:
Siglec-7/9 as Innate Immune Checkpoints: Siglec-7 and Siglec-9 function as natural inhibitory receptors that prevent overactivation of innate immune cells driving transplant rejection. Patients with higher levels of these receptors show improved allograft survival, suggesting a protective role [48].
Preclinical Models: In murine models, deficiency of Siglec-E (the mouse counterpart of human Siglec-7 and Siglec-9) accelerates acute rejection and increases inflammation across heart, kidney, and skin transplantation models [48].
Table 3: Quantitative Findings on GlycoRNA/Siglec in Disease Models
| Disease Context | Key Findings | Experimental Evidence |
|---|---|---|
| Glioma | GlycoRNA depletion inhibits cell viability and proliferation | CCK-8 and Ki67 assays showed significant reduction after glycoRNA removal [2] |
| Breast Cancer | Siglec-7 upregulated in tumors vs. normal tissue | Transcript analysis in 45 patients showed significant increase [50] |
| Breast Cancer | High Siglec-7 correlates with poor prognosis | Association with negative ER/PR status and advanced grade [50] |
| Transplant Rejection | Siglec-7/9 associated with improved graft survival | Human transplant biopsies showed correlation with outcomes [48] |
| Transplant Models | Siglec-E deficiency accelerates rejection | Mouse models of heart, kidney, skin transplantation [48] |
Experimental Protocols for Key GlycoRNA Studies
Protocol 1: GlycoRNA Isolation and Detection via Metabolic Labeling
This fundamental protocol adapted from published studies [2] enables researchers to isolate and detect glycoRNAs from cultured cells:
Cell Culture and Metabolic Labeling:
- Culture cells (e.g., glioma lines U87/LN229, HeLa) in appropriate medium.
- Treat with 50-100 μM Ac4ManNAz in DMSO for 24 hours; include vehicle-only controls.
- Maintain standard culture conditions (37°C, 5% CO₂) during labeling.
RNA Extraction:
- Lyse cells using TRIzol reagent or similar.
- Perform organic phase separation with chloroform.
- Precipitate RNA with isopropanol, wash with ethanol, and resuspend in RNase-free water.
Click Chemistry Conjugation:
- React RNA samples with DBCO-biotin (50-100 μM) in PBS for 1-2 hours at room temperature.
- Remove excess DBCO-biotin using ethanol precipitation or column purification.
Detection and Analysis:
- Separate RNA by denaturing gel electrophoresis.
- Transfer to membranes and probe with streptavidin-HRP for chemiluminescent detection.
- Confirm RNA nature by RNase sensitivity controls.
Protocol 2: Assessing Functional Roles of GlycoRNA in Cell Proliferation
This protocol outlines approaches to evaluate the contribution of glycoRNAs to cellular proliferation [2]:
GlycoRNA Depletion:
- Enzymatic approach: Treat live cells with sialidase (0.1-1 U/mL) or PNGase F to remove cell surface glycoRNAs.
- Genetic approach: Use siRNA or CRISPR to target key glycosylation enzymes (OST complex components).
Functional Assays:
- CCK-8 Assay: Seed cells in 96-well plates (3,000-5,000 cells/well), add CCK-8 reagent at various timepoints, and measure absorbance at 450nm.
- Ki67 Staining: Fix cells, permeabilize, stain with anti-Ki67 antibodies, and quantify positive cells via flow cytometry.
- TUNEL Assay: Fix cells and assess apoptosis using terminal deoxynucleotidyl transferase dUTP nick end labeling.
Data Analysis:
- Compare proliferation rates between glycoRNA-depleted and control cells.
- Perform statistical analysis (t-tests, ANOVA) to determine significance.
- Correlate glycoRNA levels with functional outcomes.
The Scientist's Toolkit: Essential Research Reagents
Table 4: Key Research Reagents for GlycoRNA Studies
| Reagent/Category | Specific Examples | Function/Application |
|---|---|---|
| Metabolic Labelers | Ac4ManNAz, Ac4GalNAz | Incorporate bioorthogonal handles into glycans for detection [2] |
| Click Chemistry Reagents | DBCO-biotin, Azide-fluorophores | Covalently tag labeled glycoconjugates for detection/purification [2] |
| Enzymes | Sialidase, PNGase F, Endo F2/F3, RNase Cocktail | Characterize glycoRNA sensitivity and composition [2] |
| Detection Systems | Streptavidin-HRP, Anti-biotin antibodies | Visualize and quantify glycoRNAs after separation [2] |
| Siglec Reagents | Siglec-Fc chimeras, Anti-Siglec antibodies | Study glycoRNA-Siglec interactions and binding [1] [6] |
| Molecular Biology Kits | RNA extraction kits, Small RNA seq kits | Isolate and analyze glycoRNA components [2] |
Therapeutic Implications and Future Directions
Therapeutic Targeting Strategies
The glycoRNA-Siglec axis presents multiple opportunities for therapeutic intervention:
Siglec-Targeted Immunotherapies: Antibodies that block inhibitory Siglecs (e.g., Siglec-7, Siglec-9) could enhance anti-tumor immunity by relieving immunosuppression [50] [47]. Conversely, agonists of these receptors might ameliorate autoimmune conditions or transplant rejection [48].
GlycoRNA-Directed Approaches: Antisense oligonucleotides targeting the RNA moieties of pathogenic glycoRNAs offer a highly specific intervention strategy [25]. The extracellular accessibility of glycoRNAs circumvents delivery challenges associated with intracellular targets.
Glycoengineering Strategies: Modifying the glycan components of glycoRNAs through metabolic engineering or enzymatic approaches could alter their receptor binding properties and functional outcomes [46].
Diagnostic and Prognostic Applications
Biomarker Potential: GlycoRNAs detected in extracellular vesicles or circulating in biofluids may serve as diagnostic or prognostic markers for cancer, autoimmune diseases, and other conditions [1] [25].
Patient Stratification: Siglec expression patterns, such as elevated Siglec-7 in breast cancer, may identify patients who would benefit from specific immunotherapy approaches [50].
Knowledge Gaps and Future Research Priorities
Despite rapid progress, significant questions remain unanswered:
Complete Biosynthetic Pathway: The detailed mechanisms by which RNAs access the glycosylation machinery and the full complement of required enzymes need elucidation [1] [6].
Structural Basis of Interactions: High-resolution structures of glycoRNA-Siglec complexes would inform rational drug design [6].
Physiological and Pathological Functions: The roles of glycoRNAs in normal physiology and across different disease states remain largely unexplored [46].
Therapeutic Development: Optimizing strategies to specifically target disease-relevant glycoRNA-Siglec interactions without disrupting beneficial functions requires further investigation [47].
The discovery of glycoRNAs has unveiled a previously unrecognized dimension of immune regulation, challenging long-standing paradigms in both RNA biology and glycobiology. These unique biomolecules, through their interactions with Siglec receptors and potentially other immune recognition systems, represent a novel mechanism of cellular communication and immunomodulation. The emerging evidence implicates glycoRNA-Siglec interactions in diverse pathological processes including cancer progression, autoimmune pathogenesis, and transplant rejection.
Methodological advances in glycoRNA detection, visualization, and functional characterization are accelerating our understanding of these molecules. As research in this field progresses, the therapeutic potential of targeting the glycoRNA-Siglec axis continues to grow, offering promising avenues for biomarker development, immune modulation, and precision medicine. Future studies elucidating the structural details, biosynthetic mechanisms, and disease-specific functions of glycoRNAs will undoubtedly uncover new opportunities for therapeutic intervention across a spectrum of human diseases.
Glycosylated RNAs (glycoRNAs) represent a groundbreaking discovery at the intersection of RNA biology and glycobiology, challenging long-standing paradigms of cellular compartmentalization [6] [5]. These molecules, consisting of small non-coding RNAs covalently modified with sialylated glycans, have been identified on the cell surface where they potentially mediate critical intercellular communication and immune recognition processes [1] [25]. However, the investigation of their tissue and context-specific functions presents unique methodological challenges that must be addressed to advance the field. The inherent diversity of RNA species, glycan structures, and their combinatorial pairing across different biological contexts creates a complex landscape requiring sophisticated analytical approaches [27] [51]. This technical guide examines current methodologies for addressing tissue and context specificity in glycoRNA research, providing experimental frameworks for researchers exploring these novel biomolecules in health and disease.
Technical Foundations: Analytical Approaches for Tissue and Context-Resolved GlycoRNA Profiling
Detection and Enrichment Strategies
The accurate detection and enrichment of glycoRNAs is a prerequisite for any tissue or context-specific functional study. Multiple complementary approaches have been developed, each with distinct advantages and limitations for specific experimental applications.
Table 1: GlycoRNA Detection and Enrichment Methodologies
| Method | Principle | Applications | Sensitivity | Tissue Compatibility |
|---|---|---|---|---|
| Metabolic Labeling with Click Chemistry [27] [25] | Incorporation of azide-modified sugar precursors (Acâ‚„ManNAz) into glycans, followed by bioconjugation with biotin-alkyne probes | In vitro systems, cell culture, mechanistic studies | High with amplification | Limited to viable, metabolically active cells |
| Periodate Oxidation and Aldehyde Ligation (rPAL) [1] [27] | Selective oxidation of sialic acid diols to aldehydes followed by biotin-hydrazide conjugation | Native tissue samples, clinical specimens, biobank materials | High (order of magnitude improvement over metabolic labeling) [1] | Excellent for preserved tissues, blood samples [51] |
| Lectin Affinity Binding [27] | Leverages natural lectin-glycan interactions (e.g., WGA, MALII) | Glycan structure-specific isolation, interaction studies | Moderate | Compatible with various tissue lysates |
| Aptamer and RNA in situ Hybridization-mediated Proximity Ligation Assay (ARPLA) [1] [27] | Dual recognition of glycans and RNA with proximity ligation and rolling circle amplification | Single-cell imaging, spatial mapping, heterogeneous tissues | Single-molecule detection | Optimal for fixed cells and tissue sections |
The selection of an appropriate enrichment strategy fundamentally shapes the scope and resolution of subsequent tissue-specific analyses. Metabolic labeling approaches provide exceptional sensitivity but are restricted to systems amenable to precursor incorporation, while chemical biology tools like rPAL enable investigation of native glycoRNAs in diverse tissue contexts including archived clinical specimens [1] [51]. For spatial resolution at the cellular level, imaging techniques such as ARPLA offer single-molecule detection capability while preserving tissue architecture information [1].
Sequencing and Analytical Frameworks
Following enrichment, advanced sequencing approaches enable comprehensive profiling of the RNA components of glycoRNAs across different biological contexts. GlycoRNA-seq represents a specialized methodology optimized for capturing glycosylated small non-coding RNAs that would be undetectable by conventional RNA sequencing [51]. The experimental workflow typically proceeds through: (1) sample preparation with appropriate enrichment; (2) library construction specifically designed for small RNAs; (3) high-throughput sequencing; and (4) specialized bioinformatic analysis.
Key analytical considerations for tissue and context-specific studies include the development of appropriate normalization strategies to account for varying glycoRNA abundance across tissues, and the implementation of comparative frameworks that can identify statistically significant differences in glycoRNA profiles between biological conditions [51]. Bioinformatics tools such as GlyinsRNA, which applies machine learning to predict glycosylation sites on RNA molecules, can provide valuable insights into potential functional domains that may exhibit tissue-specific regulation [27].
Figure 1: Experimental workflow for tissue-specific glycoRNA profiling, highlighting critical methodological decision points.
Tissue-Specific Functional Studies: Methodological Considerations
Context-Dependent GlycoRNA-Protein Interactions
A principal mechanism through which glycoRNAs exert tissue and context-specific functions is via interactions with specific receptor proteins, particularly members of the sialic acid-binding immunoglobulin-type lectin (Siglec) family [1] [14] [25]. Functional studies of these interactions require specialized approaches that account for the unique biophysical properties of both components.
Immunological Assays for Receptor Engagement: Flow cytometry-based binding assays using soluble Siglec-Fc chimeric proteins enable quantification of glycoRNA-receptor interactions on intact cells while preserving native presentation [6] [25]. This approach maintains the tissue-specific context of glycoRNA display, including potential co-receptor requirements and membrane microdomain localization. For example, studies have identified that from 12 human Siglec-Fc reagents tested, Siglec-11 and Siglec-14 demonstrate binding vulnerable to RNase A treatment, suggesting specific recognition of glycoRNA ligands [6]. Additionally, neutrophil recruitment assays have revealed that glycoRNA-mediated interactions with P-selectin on endothelial cells contribute to inflammatory responses in a tissue-specific manner [1].
Genetic and Pharmacological Perturbation: Establishing causal relationships between glycoRNA expression and functional outcomes requires targeted perturbation approaches. CRISPR-Cas9 mediated knockout of specific glycosyltransferases or RNA-modifying enzymes in tissue-specific model systems can elucidate biosynthetic requirements across different cellular contexts [27] [25]. Similarly, pharmacological inhibitors targeting key enzymes in the glycan biosynthesis pathway (e.g., NGI-1 for oligosaccharyltransferase, kifunensine for mannosidase I) enable acute and reversible manipulation of glycoRNA expression to assess functional consequences in tissue-specific models [27].
Experimental Models for Tissue Context
The biological context in which glycoRNAs are studied significantly influences observed functional outcomes. Several model systems offer complementary advantages for tissue-specific investigations.
Primary Cell Systems: Isolation and study of glycoRNAs from primary human cells, such as human umbilical vein endothelial cells (HUVECs) or human primary alveolar epithelial cells (hPAEpCs), maintains native tissue-specific expression patterns and functional associations [1] [14]. These systems are particularly valuable for validating findings from immortalized cell lines and establishing clinical relevance.
Genetic Model Organisms: Murine models provide opportunities to investigate glycoRNA function in complex tissue environments and physiological contexts. The conservation of glycoRNA biosynthetic machinery between humans and mice enables translational studies, though species-specific differences in both RNA expression and glycan structures must be carefully considered in experimental design [9].
Table 2: Research Reagent Solutions for GlycoRNA Functional Studies
| Reagent Category | Specific Examples | Function in Experimental Design | Tissue/Context Applications |
|---|---|---|---|
| Metabolic Labels | Acâ‚„ManNAz [27] | Incorporates azide-modified sialic acid precursors into nascent glycans | Cell culture systems, ex vivo organ cultures |
| Enzymatic Tools | PNGase F, Sialidase, Endo glycosidases [27] [25] | Selective removal of specific glycan structures to probe functional contributions | Glycan structure-function studies across tissues |
| Click Chemistry Reagents | DBCO-biotin [27] | Copper-free conjugation for biotin tagging of azide-labeled glycoRNAs | Enrichment and detection across tissue types |
| Immune Reagents | Siglec-Fc chimeric proteins [6] [25] | Detection probes for receptor binding studies | Immune cell recognition assays, tissue-specific ligand screening |
| Pharmacological Inhibitors | NGI-1 (OST inhibitor), Kifunensine (mannosidase I inhibitor) [27] [25] | Acute disruption of glycoRNA biogenesis | Functional perturbation studies in diverse cellular contexts |
Tissue-Specific GlycoRNA Functions in Physiological and Pathological Contexts
Immune Regulation and Inflammation
GlycoRNAs demonstrate significant tissue-specific functions in immune regulation, particularly through their interactions with Siglec receptors that exhibit distinct expression patterns across immune cell types [6] [1] [14]. In myeloid cells, glycoRNAs have been identified as ligands for Siglec-11 and Siglec-14, potentially modulating inflammatory responses in a tissue-specific manner [6]. Functional studies in neutrophils have revealed that glycoRNAs contribute to recruitment to inflammatory sites through interactions with P-selectin on endothelial cells, with demonstrated dependence on the Sidt gene family for proper expression and function [1]. These findings highlight the importance of studying glycoRNA function in specific immune cell populations and inflammatory contexts to fully understand their contributions to immunity.
Cancer and Disease-Specific Expression
Aberrant glycosylation is a well-established hallmark of cancer, and growing evidence suggests that glycoRNAs contribute to disease pathogenesis in a tissue-specific manner [1] [41]. Different cancer types display distinct glycoRNA profiles that may serve as potential biomarkers or therapeutic targets. For instance, studies using the ARPLA imaging technique have revealed differential expression of specific glycoRNAs (U1, U35a, and Y5) across breast cancer cell lines with varying tumorigenic potential [27]. Similarly, glycosylated Y-RNAs are of particular interest due to their association with autoantigens in systemic lupus erythematosus (SLE), suggesting tissue-specific presentation may contribute to loss of immune tolerance [25].
Figure 2: GlycoRNA-mediated immunomodulatory pathways showing tissue and context-specific interactions with Siglec receptors and P-selectin.
Future Directions: Advancing Tissue and Context Resolution in GlycoRNA Research
The field of glycoRNA biology stands to benefit tremendously from continued technological innovations that enhance resolution of tissue and context-specific functions. Several promising avenues are emerging that will address current limitations:
Spatial Omics Integration: Combining glycoRNA detection with spatial transcriptomics and glycomics approaches will enable precise mapping of these molecules within tissue architecture, revealing microenvironment-specific expression patterns and functions [1]. This is particularly relevant for understanding heterogeneous tissues such as tumors where stromal and immune cell interactions create complex functional niches.
Single-Cell GlycoRNA Profiling: Adapting current methodologies to single-cell resolution will uncover cellular heterogeneity in glycoRNA expression and function within tissues [27]. Recent advances in sensitive detection methods like ARPLA provide a foundation for these developments, potentially enabling classification of cell states based on glycoRNA profiles in addition to conventional transcriptomic signatures.
Mechanistic Studies of Biosynthesis: A critical unanswered question in the field concerns the subcellular site and precise mechanisms of RNA glycosylation, particularly given the apparent disconnection between canonical RNA localization and the ER-Golgi localization of glycosylation machinery [6] [25]. Tissue-specific variations in this process may exist and contribute to functional diversity. Developing tools to visualize and manipulate the biosynthesis machinery in specific tissue contexts will be essential for understanding how glycoRNA expression is regulated across different biological systems.
As these methodological advances mature, they will undoubtedly reveal new dimensions of tissue and context specificity in glycoRNA biology, providing unprecedented insights into the functional significance of these novel biomolecules in health and disease.
For decades, the landscape of cellular glycosylation was dominated by two principal scaffolds: proteins and lipids. The discovery in 2021 of glycosylated RNA (glycoRNA) – small, non-coding RNAs modified with complex N-glycans and displayed on the cell surface – fundamentally challenged this paradigm [1] [6] [43]. This finding emerged from the unexpected convergence of RNA biology and glycoscience, two fields previously thought to operate in distinct cellular compartments [43]. GlycoRNAs are now established as a third major class of glycoconjugates, primarily consisting of small nuclear RNAs (snRNAs), small nucleolar RNAs (snoRNAs), transfer RNAs (tRNAs), and Y RNAs modified with sialylated and fucosylated N-glycans [1] [25]. Their predominant localization on the outer leaflet of the plasma membrane positions them as potential key players in intercellular communication and immune recognition, suggesting functions that extend far beyond the traditional roles of RNA [6] [43]. This whitepaper synthesizes the current state of glycoRNA research, detailing its molecular architecture, detection methodologies, confirmed and hypothesized biological roles, and the critical experimental tools required to advance the field from structural characterization to definitive functional understanding.
Molecular Architecture of GlycoRNA
The molecular structure of glycoRNA represents one of the most pressing and recently elucidated knowledge gaps. The central question has been the nature of the covalent linkage between the RNA molecule and the complex N-glycan.
The acp³U Linkage and Glycan Composition
A significant breakthrough in 2024 identified the modified RNA base 3-(3-amino-3-carboxypropyl)uridine (acp³U) as the primary attachment site for N-glycans on RNA [1] [52] [25]. acp³U is a highly conserved modified uridine found in bacterial and mammalian tRNAs, known to enhance tRNA thermostability [1]. The chemical linkage via this residue provides the robustness needed to withstand stringent biochemical purification, including organic phase separation and silica-based RNA purification [25]. The attached glycans are not simple monosaccharides but complex N-glycans rich in sialic acid and fucose, structurally analogous to those found on glycoproteins [1] [25]. This composition is consistent with the observed binding to Siglec receptors, which recognize sialic acid caps [43].
Biosynthetic Pathway: An Endoplasmic Reticulum Connection
The biosynthesis of glycoRNA remains incompletely mapped but involves a surprising overlap with the canonical N-glycosylation machinery. Key evidence indicates that the oligosaccharyltransferase (OST) complex, which catalyzes protein N-glycosylation in the endoplasmic reticulum (ER), is also essential for glycoRNA production [1] [6]. Genetic or pharmacological inhibition of OST diminishes glycoRNA levels [6] [25]. This dependency creates a fascinating cell biological paradox: the established sites of N-glycan assembly and maturation are the ER and Golgi apparatus, compartments from which RNA is conventionally excluded. Resolving how RNA substrates access this biosynthetic machinery is a primary focus of ongoing research. The prevailing hypothesis is that the process resembles protein N-glycosylation and may even utilize identical enzymes [1].
Table 1: Key Characteristics of the GlycoRNA Molecular Structure
| Structural Feature | Description | Functional Implication |
|---|---|---|
| RNA Backbone | Small non-coding RNAs (e.g., Y RNAs, snRNAs, snoRNAs) [1] [6] | Potential link to known non-coding RNA functions and disease associations (e.g., autoimmunity) |
| Glycan Type | Sialylated and fucosylated complex N-glycans [1] [25] | Recognition by lectin receptors (e.g., Siglecs); role in intercellular communication |
| Covalent Linkage | Via the acp³U (3-(3-amino-3-carboxypropyl)uridine) modified base [1] [52] | Provides biochemical stability; defines a novel RNA modification |
| Biosynthetic Machinery | Dependent on the oligosaccharyltransferase (OST) complex and the canonical N-glycan pathway [1] [6] | Links RNA biology to glycosylation compartments (ER/Golgi); suggests a conserved mechanism |
Diagram 1: Basic structure of glycoRNA showing the acp³U linkage.
Advanced Methodologies for Detection and Analysis
The study of glycoRNA demands specialized techniques capable of detecting the unique hybrid nature of these molecules. Standard RNA sequencing protocols are insufficient as they do not preserve or report glycan modifications.
Metabolic Labeling and Click Chemistry
The initial discovery of glycoRNA relied on metabolic labeling with peracetylated N-azidoacetylmannosamine (Ac4ManNAz) [9]. This bioorthogonal sugar precursor is incorporated into nascent sialic acid-containing glycans. Following RNA extraction, a click chemistry reaction (e.g., strain-promoted azide-alkyne cycloaddition) attaches a biotin reporter to the azide-labeled glycans, enabling the affinity purification and detection of glycoRNAs via streptavidin-based probes like Northern blotting [9] [25]. This protocol is foundational but requires rigorous controls to rule out co-purifying contaminants.
Refined Enrichment and Visualization Techniques
Recent advances have produced more specific and sensitive tools:
- RNA-optimized Periodate oxidation and Aldehyde Labeling (rPAL): This method leverages the unique reactivity of 1,2-diols in sialic acids. Periodate oxidation generates aldehyde groups that form stable oxime bonds with aminooxy-functionalized solid-phase supports, enabling specific labeling and enrichment of glycoRNAs. When coupled with high-sensitivity mass spectrometry, rPAL was instrumental in identifying acp³U as the glycan attachment site [1].
- Dual-recognition Fluorescence Resonance Energy Transfer (drFRET): An imaging technology that allows for the visualization of glycosylated RNAs on small extracellular vesicles (sEVs) derived from cancer cell lines and clinical serum samples. drFRET has been used to elucidate interactions between glycoRNAs and proteins like Siglec-10, Siglec-11, and P-selectin [1].
- Sialic Acid Aptamer and RNA in situ Hybridization-mediated Proximity Ligation Assay (ARPLA): This technique achieves high-sensitivity visualization of glycoRNAs at the single-cell level. It uses dual recognition of glycans and RNA to trigger an in situ ligation reaction, followed by rolling circle amplification for signal output. ARPLA revealed that glycoRNAs undergo intracellular trafficking via SNARE protein-mediated secretory exocytosis [1].
Table 2: Core Methodologies for GlycoRNA Analysis
| Method | Principle | Key Application | Critical Consideration |
|---|---|---|---|
| Metabolic Labeling & Click Chemistry [9] [25] | Incorporation of "clickable" sugar precursors (e.g., Ac4ManNAz) into glycans | Initial discovery and bulk detection | Potential for glycoprotein contamination; requires proteinase K controls under denaturing conditions [9] |
| rPAL (RNA-optimized Periodate oxidation and Aldehyde Labeling) [1] | Specific oxidation of sialic acid diols for covalent capture and MS analysis | Defining glycan-RNA linkage (e.g., identification of acp³U) | High specificity for native sialylated structures |
| drFRET (Dual-recognition FRET) [1] | FRET signal requires simultaneous presence of glycan and RNA epitopes | Visualizing glycoRNAs on extracellular vesicles in biofluids | Enables analysis in complex clinical samples (e.g., serum) |
| ARPLA (Aptamer & RNA in situ Hybridization-mediated Proximity Ligation Assay) [1] | Proximity ligation and rolling circle amplification for signal boost | Single-cell imaging and spatial mapping of glycoRNA | Reveals subcellular trafficking and nanoscale organization |
Diagram 2: Core workflow for glycoRNA detection and analysis.
Critical Experimental Controls and Controversies
The field is maturing with a growing emphasis on methodological rigor. A significant 2025 study highlighted that glycoproteins can co-purify with small RNA preparations using standard protocols, representing a considerable source of potential contamination [9]. These glycosylated molecules showed resistance to RNase A/T1 but were sensitive to proteinase K digestion under denaturing conditions [9]. This finding necessitates the implementation of stringent controls, including:
- Proteinase K treatment in denaturing Tris buffer (DTB) to ensure complete protein digestion [9].
- The use of orthogonal detection methods (e.g., combining rPAL and drFRET) to confirm findings.
- Careful interpretation of data, particularly for RNase sensitivity assays where purification steps post-digestion can influence results [9].
Elucidating Biological Function: From Ligands to Therapeutic Targets
The positioning of glycoRNAs on the cell surface strongly implies a role in extracellular communication. Research is now converging on several specific biological functions, particularly in immune regulation and disease pathogenesis.
Role as Siglec Ligands in Immune Regulation
A leading hypothesis posits that glycoRNAs serve as ligands for the Siglec (sialic acid-binding immunoglobulin-like lectin) family of immunoregulatory receptors [1] [6] [43]. Of the 12 human Siglec-Fc reagents tested, nine bind to HeLa cells, with the binding of Siglec-11 and Siglec-14 being particularly vulnerable to RNase treatment [6] [43]. This suggests that glycoRNAs are direct ligands for specific Siglecs. Given that Siglecs are known to function as immune checkpoints and are implicated in tumor immune evasion [1], glycoRNAs may represent a novel class of self-ligands that fine-tune immune responses. Dysregulation of this interaction could be a factor in autoimmune diseases [43].
Functional Roles in Inflammation and Cancer
Beyond Siglec binding, functional studies are linking glycoRNAs to specific physiological and pathological processes:
- Neutrophil Recruitment: GlycoRNAs enhance neutrophil recruitment to inflammatory sites in vivo by interacting with P-selectin on endothelial cells, a process dependent on the Sidt gene [1] [28].
- Cell-Penetrating Peptide (CPP) Entry: Recent work has revealed that glycoRNAs form nanoclusters on the cell surface with cell-surface RNA-binding proteins (csRBPs) like nucleolin and La protein. These glycoRNA-csRBP clusters act as gateways for the entry of positively charged cell-penetrating peptides (e.g., HIV-1 TAT) [52]. Removing surface RNA greatly reduces the cellular uptake efficiency of these peptides [52].
- Cancer Biomarker Potential: GlycoRNAs have been detected in multiple human cell lines and share homology with disease-associated small RNAs [1]. Their presence on small extracellular vesicles (sEVs) in cancer cell lines and clinical sera makes them attractive, accessible targets for non-invasive biomarker development [1] [28].
Diagram 3: Key biological functions of cell-surface glycoRNA.
Advancing glycoRNA research requires a suite of specialized reagents, tools, and databases. The following table details essential resources for designing a rigorous research program.
Table 3: Essential Research Reagents and Resources for GlycoRNA Investigation
| Resource Category | Specific Examples / Items | Function and Application |
|---|---|---|
| Metabolic Labelers | Peracetylated N-azidoacetylmannosamine (Ac4ManNAz) [9] | Introduces a bioorthogonal azide tag into sialic acid residues of nascent glycans for subsequent click chemistry conjugation. |
| Click Chemistry Reagents | DBCO-Biotin, SPAAC reagents [9] [25] | Strain-promoted azide-alkyne cycloaddition (SPAAC) reagents for covalently linking a biotin reporter or fluorophore to metabolically labeled glycans. |
| Enzymatic Tools | RNase A/T1 cocktail, Proteinase K (with/without denaturing buffer), PNGase F [9] [25] | Used for critical control experiments: RNase sensitivity confirms RNA presence; Proteinase K under denaturing conditions rules out glycoprotein contamination; PNGase F cleaves N-glycans. |
| Affinity Purification | Streptavidin-coated Beads/Magnetic Resins [9] | For pull-down of biotin-tagged glycoRNAs after click chemistry, enabling enrichment from complex lysates. |
| Imaging Probes | Fluorescently labeled oligonucleotides (for FISH), Lectins, Siglec-Fc chimeric proteins [1] [6] | For spatial mapping: Oligonucleotides target RNA sequence; Lectins/Siglecs target the glycan moiety. ARPLA and drFRET combine these principles. |
| Bioinformatic Databases | GlycoRNAdb (http://www.glycornadb.com) [16] | A curated database of glycoRNA sequences, structures, abundance, and glycan information across tissues and cell lines; enables homology searches (BLAST) and data exploration. |
| Cell Engineering Tools | CRISPR-Cas9 for KO of OST subunits (e.g., STT3A/B) or glycan biosynthetic genes (e.g., GALE) [25] | Genetic perturbation of the glycosylation machinery to establish its necessity for glycoRNA biogenesis and to study functional consequences. |
The discovery of glycoRNA has irrevocably expanded the central dogma of molecular glycosylation. From a fundamental science perspective, the immediate future requires a concerted effort to resolve the persistent knowledge gaps, most notably the complete elucidation of the biosynthetic pathway that allows RNA to be glycosylated by ER/Golgi-resident machinery. Furthermore, the precise structural nuances of the acp³U-glycan linkage and the full repertoire of its binding partners beyond Siglecs and P-selectin remain to be fully characterized.
For translational researchers and drug development professionals, the implications are profound. GlycoRNAs represent a new universe of druggable cell-surface targets and a potential source of non-invasive biomarkers detectable in extracellular vesicles from biofluids [1] [28]. Their involvement in immune cell recruitment and viral peptide entry opens avenues for therapeutic intervention in cancer, autoimmune diseases, and infectious diseases [52] [43] [28]. As the toolset for studying these molecules—from ARPLA imaging to GlycoRNAdb—continues to mature and become more accessible, the field is poised to move from foundational discovery to functional validation and therapeutic exploration. The journey to bridge the knowledge gaps from molecular structure to definitive biological roles is well underway, promising to reshape our understanding of cell biology and open new frontiers in medicine.
Establishing Significance: Validating GlycoRNA as a Crucial Biological Player
Glyco-conjugates, molecules composed of carbohydrates covalently linked to other biological scaffolds, are fundamental to numerous cellular processes. Traditionally, the study of cellular glycosylation has focused on two primary conjugates: glycoproteins and glycolipids. Glycoproteins, proteins modified with glycans, play critical roles in protein folding, stability, and cell-cell recognition. Glycolipids, lipids attached to sugar moieties, are essential components of the cell membrane and contribute to its structural integrity and signaling capabilities. The discovery of a third scaffold, glycosylated RNA (glycoRNA), represents a paradigm shift in glycobiology [53]. This novel class of biomolecules, where sialylated glycans are covalently linked to small non-coding RNAs, suggests a direct and previously unknown interface between RNA biology and glycobiology [53]. This technical guide provides a comparative analysis of these three glyco-conjugates, focusing on their structural bases, biosynthetic pathways, functional roles, and the experimental methodologies used in their study, framed within the context of advancing glycoRNA research.
Structural and Biochemical Foundations
The fundamental distinction between glycoRNA, glycoproteins, and glycolipids lies in their core biochemical scaffolds. The following table summarizes their key structural characteristics.
Table 1: Comparative Structural Foundations of Glyco-Conjugates
| Feature | GlycoRNA | Glycoproteins | Glycolipids |
|---|---|---|---|
| Core Scaffold | Small non-coding RNAs (e.g., Y RNAs, snRNAs) [53] [2] | Proteins | Lipids (e.g., sphingolipids, ceramides) |
| Primary Glycan Type | N-glycans (sialylated, fucosylated) [53] [2] | N-glycans, O-glycans, GPI-anchors | Oligosaccharides, gangliosides |
| Key Linkage / Site | Modified base acp3U (3-(3-amino-3-carboxypropyl)uridine) [54] [52] | Asparagine (N-linked), Serine/Threonine (O-linked) | Glycosidic bond to lipid backbone |
| Cellular Localization | Predominantly cell surface [53] [52] | Secreted, membrane-bound, intracellular | Cell membrane (primarily outer leaflet) |
A critical breakthrough in glycoRNA research was the identification of acp3U as the specific RNA base that serves as the attachment point for N-glycans [54] [52]. This modification provides a defined chemical linkage distinct from the proteinaceous linkages of traditional glycoconjugates. Furthermore, the glycan structures on glycoRNAs are not random; they are enriched in sialic acid and fucose and depend on the canonical N-glycan biosynthetic machinery, indicating a shared biosynthetic origin with glycoproteins but a unique destination [53].
Biosynthesis and Cellular Processing
The biosynthesis of glyco-conjugates involves complex enzymatic pathways. Evidence indicates that glycoRNA assembly depends on canonical N-glycan biosynthetic machinery [53], suggesting a convergence with glycoprotein synthesis in the endoplasmic reticulum and Golgi apparatus. However, the mechanism by which RNA is shuttled into these compartments for glycosylation remains a central question. In contrast, glycolipid synthesis follows a distinct, lipid-centric pathway.
The following diagram illustrates the conceptual workflow for the metabolic labeling and detection of glycoRNA, a key technique for probing its biosynthesis and presence.
Fig 1. Metabolic Labeling Workflow for GlycoRNA: This diagram outlines the key steps for labeling and detecting glycoRNAs using metabolic precursors like Ac4ManNAz, which is converted into azido-sialic acid and incorporated into glycans on RNA.
Localization and Biological Functions
The cellular localization and biological functions of these conjugates highlight their diverse roles. Glycoproteins and glycolipids are well-established players in cell adhesion, signaling, and as receptors. The discovery that the majority of glycoRNAs are present on the cell surface has expanded the potential scope of RNA in extracellular biology [53]. These cell-surface glycoRNAs can interact with pattern recognition receptors and other immune molecules.
Table 2: Comparative Localization and Functions of Glyco-Conjugates
| Attribute | GlycoRNA | Glycoproteins | Glycolipids |
|---|---|---|---|
| Primary Localization | Cell surface [53] | Cell surface, secreted, intracellular | Cell membrane |
| Key Biological Functions | Immune regulation (e.g., Siglec, P-selectin binding) [21] [14], cell communication [10] | Enzymatic catalysis, structural support, cell signaling, immunity | Membrane organization, signal transduction, cell adhesion |
| Role in Disease | Glioma proliferation [2], neutrophil recruitment [21], autoimmunity [14] | Cancer metastasis, congenital disorders of glycosylation (CDGs) | Lysosomal storage diseases, cancer |
| Ligands/Interactions | Siglec family, P-selectin [53] [21] | Lectins, antibodies, other proteins | Toxins, viruses, antibodies |
A significant functional role of glycoRNAs is their involvement in immune regulation. They have been shown to interact with Siglec receptors and P-selectin on endothelial cells, facilitating processes such as neutrophil recruitment to inflammatory sites [21] [14]. Furthermore, in the context of cancer, glycoRNAs are abundant on small extracellular vesicles (sEVs) and have been profiled for cancer diagnostics with high accuracy [21]. Functional studies in glioma cells demonstrate that depleting cell-surface glycoRNAs significantly inhibits glioma cell viability and proliferation, underscoring their potential as a therapeutic target [2].
The following diagram maps the known and proposed biological interactions and functions of cell-surface glycoRNA.
Fig 2. GlycoRNA Cell-Surface Functions: This diagram summarizes the key biological interactions and roles of glycoRNAs, including immune recognition, involvement in disease processes, and structural associations.
Experimental Methodologies and Techniques
The study of glycoRNA requires specialized and rigorous protocols to isolate and characterize this novel conjugate, often building upon and modifying methods used for glycoprotein analysis.
Key Protocols for GlycoRNA Isolation and Detection
The foundational protocol for glycoRNA extraction involves a stringent process to ensure high purity and eliminate contamination from glycoproteins or glycolipids [9] [53]. The core steps are:
- Metabolic Labeling: Cells are treated with peracetylated N-azidoacetylmannosamine (Ac4ManNAz), a precursor that is metabolically converted into azide-modified sialic acid and incorporated into nascent glycans [53] [2].
- Stringent RNA Extraction: RNA is isolated using TRIzol (acid phenol and guanidine salts) and subjected to a series of purification steps, including ethanol precipitation and desalting via silica columns (e.g., Zymo Spin columns) [9] [53].
- Protein Degradation: A critical step involves digesting the sample with a high concentration of proteinase K to remove any contaminating glycoproteins. A recent study highlights that performing this digestion under denaturing conditions is crucial for complete protein removal, as resistant glycoproteins like LAMP1 can be a source of glycans in RNA preparations [9].
- Click Chemistry Conjugation: The purified RNA is reacted with a dibenzocyclooctyne (DBCO)-biotin probe via copper-free click chemistry, which specifically tags the azide-containing glycans [53] [2].
- Detection and Analysis: The biotinylated glycoRNA is then detected by denaturing gel electrophoresis followed by Northern blotting with a streptavidin probe [53]. For functional studies, this allows for subsequent analysis like RNA sequencing to identify the specific RNA species or mass spectrometry to analyze the glycan structures [2].
Advanced and Emerging Detection Techniques
Beyond the foundational protocol, several advanced methods have been developed:
- Dual Recognition FRET (drFRET): This sensitive technique uses two distinct DNA probes—one for the glycan (Neu5Ac) and one for the RNA sequence—enabling specific imaging and profiling of glycoRNAs on small extracellular vesicles (sEVs) with applications in cancer diagnostics [21].
- Sequence-Specific Capture: A magnetic bead system can be developed to enrich for specific glycoRNAs (e.g., U2, U4) for downstream compositional analysis [2].
The Scientist's Toolkit: Essential Research Reagents
The following table details key reagents and their functions essential for conducting glycoRNA research.
Table 3: Essential Research Reagents for GlycoRNA Studies
| Reagent / Tool | Function / Application | Key Characteristic |
|---|---|---|
| Ac4ManNAz (Metabolic Chemical Reporter) | Labels sialic acid-containing glycans metabolically [53] [2] | Precursor for "clickable" azido-sialic acid. |
| DBCO-biotin/DBCO-PEG4-biotin | Click chemistry probe for biotinylating azide-labeled glycoRNAs [53] [21] | Enables detection and pulldown via streptavidin. |
| Proteinase K | Digests and removes contaminating proteins from RNA prep [53] | Critical for specificity; efficacy enhanced under denaturing conditions [9]. |
| Silica Columns (e.g., Zymo Spin) | Purifies and desalts RNA after extraction and click reaction [9] [53] | Removes metabolites and unconjugated reagents. |
| PNGase F / Sialidase | Glycan-cleaving enzymes used to confirm glycan identity and type on RNA [2] | Confirms protein N-glycan origin and sialylation. |
| Sequence-Specific Magnetic Beads | Enriches particular glycoRNA species (e.g., U2, U4) for targeted analysis [2] | Allows for functional study of individual glycoRNAs. |
Current Challenges and Future Directions
Despite rapid progress, the field of glycoRNA research faces several challenges. A significant concern is the potential for co-purifying glycoproteins using current protocols, which can be a considerable source of contaminating glycans and must be rigorously controlled for [9]. Furthermore, the precise enzymatic pathway responsible for attaching glycans to the acp3U base in RNA remains elusive [54].
Future research will need to:
- Elucidate the complete biosynthetic pathway of glycoRNA.
- Develop even more specific and sensitive detection methods.
- Expand the functional understanding of glycoRNA in various physiological and pathological contexts, particularly in immune regulation and cancer biology [10] [14] [2].
- Explore the therapeutic potential of targeting glycoRNAs in diseases like glioma and autoimmune disorders.
In conclusion, while glycoproteins and glycolipids remain well-established and fundamental glyco-conjugates, glycoRNA emerges as a novel and distinct player. Its unique RNA scaffold, specific glycosidic linkage, cell-surface localization, and roles in immune signaling and disease pathogenesis distinguish it from its traditional counterparts. Continued methodological refinement and functional exploration will be crucial to fully unravel the biology of this unexpected molecule.
Glycosylated RNA (glycoRNA) represents a paradigm-shifting class of biomolecules in which small, non-coding RNAs are modified with complex glycans, predominantly N-linked glycans rich in sialic acid and fucose [1] [3]. Traditionally, glycosylation was considered a modification exclusive to proteins and lipids. The discovery that RNA can also be glycosylated expands the landscape of cellular glycosylation and opens new avenues for understanding cell surface interactions [1] [6]. A defining characteristic of glycoRNAs is their localization on the outer leaflet of the plasma membrane, where they are positioned to participate in intercellular communication [1] [55] [3].
The sialic acid-binding immunoglobulin-like lectins (Siglecs) are a family of transmembrane receptors primarily expressed on immune cells. Most Siglecs are immune-inhibitory receptors that contain immunoreceptor tyrosine-based inhibitory motifs (ITIMs) and play crucial roles in modulating immune responses [56] [57]. The hypothesis that glycoRNAs may serve as novel ligands for Siglecs connects two previously disparate fields—RNA biology and glycobiology—and suggests a previously unrecognized layer of immune regulation [1] [6]. This technical guide details the experimental frameworks for validating these interactions.
Experimental Evidence: Validating GlycoRNA-Siglec Interactions
A multi-faceted approach is essential to conclusively demonstrate that glycoRNAs function as physiological ligands for Siglec receptors. Key evidence comes from binding assays, functional studies, and enzymatic validation.
Binding Affinity and Specificity
Initial binding studies have identified specific Siglec receptors capable of engaging glycoRNAs. Using soluble Siglec-Fc chimeric proteins in flow cytometry assays, researchers found that Siglec-11 and Siglec-14 exhibit glycoRNA-dependent binding to live cells, as evidenced by a significant reduction in binding after cell surface RNase treatment [1] [6]. A more recent study has identified Siglec-5 as a receptor for glycoRNAs on human monocytes [55].
Table 1: Experimentally Validated GlycoRNA-Siglec Interactions
| Siglec Receptor | Experimental System | Key Functional Readout | Citation |
|---|---|---|---|
| Siglec-11 | Siglec-Fc binding to HeLa cells; RNase sensitivity | Binding vulnerable to RNase A | [1] [6] |
| Siglec-14 | Siglec-Fc binding to HeLa cells; RNase sensitivity | Binding vulnerable to RNase A | [1] [6] |
| Siglec-5 | Flow cytometry, immunoprecipitation, Northern blot | Mediates monocyte adhesion to activated endothelial cells | [55] |
The interaction is dependent on the sialylated glycans present on glycoRNAs. Treatment of cells with sialidase, which removes terminal sialic acid residues, abolishes Siglec binding [1] [3]. Furthermore, the biosynthesis of these glycans is critical, as inhibition of the oligosaccharyltransferase (OST) complex by NGI-1 or inhibition of N-glycan trimming by kifunensine significantly diminishes glycoRNA expression and subsequent Siglec binding [1] [55].
Functional Consequences of GlycoRNA-Siglec Engagement
The biological relevance of glycoRNA-Siglec binding is underscored by functional experiments. In human monocytes, two distinct forms of glycoRNAs—glycoRNA-L (∼11 kb) and glycoRNA-S (∼0.6 kb)—have been identified [55]. Removal of these cell-surface glycoRNAs with RNase treatment significantly suppressed the adhesion of human monocytes to activated endothelial cells, a critical step in inflammation [55]. This demonstrates that glycoRNA-Siglec interactions are not merely structural but have definable physiological outcomes in immune cell trafficking.
Methodological Framework: Core Experimental Protocols
This section provides detailed protocols for key experiments validating glycoRNA-Siglec interactions.
Metabolic Labeling and Detection of GlycoRNAs
The standard method for identifying glycoRNAs relies on metabolic incorporation of azide-modified sugar precursors, enabling subsequent bioconjugation and detection.
Workflow: Metabolic Labeling & Detection
Protocol Details:
- Metabolic Labeling: Culture cells (e.g., HeLa, THP-1 monocytes) in medium containing 100 µM N-azidoacetylmannosamine-tetraacylated (Ac4ManNAz) for 40-72 hours [55] [9] [58]. Ac4ManNAz is metabolically converted into azide-modified sialic acid and incorporated into nascent glycans.
- RNA Extraction and Purification: Lyse cells in TRIzol reagent and isolate total RNA via phase separation. Precipitate the RNA from the aqueous phase with isopropanol. Further purify the RNA using silica-based spin columns (e.g., Zymo Research) to remove metabolites and unincorporated click chemistry reagents [55] [9]. To isolate the small RNA fraction (<200 nucleotides), use adjusted binding buffers [2].
- Click Chemistry Conjugation: React the purified RNA with Dibenzocyclooctyne-Polyethylene-glycol-Biotin (DBCO-PEG4-Biotin) via copper-free strain-promoted azide-alkyne cycloaddition. This covalently attaches a biotin tag to the azide-containing glycans on glycoRNAs [55] [2].
- Detection and Analysis: Separate the biotin-labeled RNA via denaturing gel electrophoresis. Transfer to a membrane and perform Northern blotting using a streptavidin-HRP probe and chemiluminescent detection. Specific glycoRNA species, such as glycoRNA-L and glycoRNA-S, can be visualized as distinct bands [55].
Critical Controls:
- Include a control without Ac4ManNAz labeling to confirm signal specificity.
- Treat an aliquot of labeled RNA with an RNase cocktail (RNase A/T1) prior to click chemistry. The signal should be abolished, confirming its RNA origin [55].
- Treat labeled RNA with PNGase F, which cleaves N-glycans. This should attenuate or eliminate the signal, confirming the presence of N-linked glycans [55].
Validating Cell Surface Localization via RNase Sensitivity
A key characteristic of glycoRNAs relevant to their function as Siglec ligands is their presence on the cell exterior.
Protocol Details:
- Harvest live, metabolically labeled cells and wash them with a balanced salt solution (e.g., HBSS).
- Resuspend the cell pellet and incubate with RNase A (e.g., 2.5-12.5 U/mL) at 37°C for 10 minutes [55].
- Wash the cells thoroughly to remove the RNase, then proceed with RNA extraction and the standard glycoRNA detection protocol (3.1).
- A significant reduction in the glycoRNA signal after extracellular RNase treatment, without loss of cell viability, confirms their exposure on the cell surface [55].
Siglec Binding Assays
A. Siglec-Fc Chimera Binding and Flow Cytometry This assay tests the binding of soluble Siglec receptors to native glycoRNAs on live cells.
Protocol Details:
- Use commercially available soluble Siglec-Fc chimeric proteins (e.g., Siglec-5-Fc, Siglec-11-Fc), which are dimers that increase binding avidity [56].
- Incubate live cells with the Siglec-Fc chimera or a control Fc protein.
- After washing, detect bound chimera using a fluorescently labeled anti-human Fc antibody and analyze by flow cytometry.
- To confirm specificity, include conditions where cells are pre-treated with RNase (as in 3.2) or sialidase to remove terminal sialic acids [1] [6]. A reduction in binding confirms the interaction is dependent on sialylated glycoRNAs.
B. Immunoprecipitation and Northern Blot This method provides biochemical evidence for a direct molecular interaction.
Protocol Details:
- Lyse cells under mild, non-denaturing conditions to preserve complexes.
- Incubate the lysate with a Siglec-Fc chimera or an antibody against the specific Siglec.
- Pull down the complex using Protein A/G beads.
- After extensive washing, recover the bound RNA from the beads.
- Analyze the eluted RNA by Northern blot (as in 3.1) or RT-qPCR to identify the specific RNA species associated with the Siglec receptor [55].
The Scientist's Toolkit: Essential Reagents and Methods
Table 2: Key Research Reagent Solutions for GlycoRNA-Siglec Studies
| Reagent / Method | Function / Purpose | Example Use Case |
|---|---|---|
| Ac4ManNAz | Metabolic chemical reporter; incorporates azide-modified sialic acid into glycans. | Initial metabolic labeling of glycoRNAs for subsequent detection [55] [2]. |
| DBCO-PEG4-Biotin | Bioorthogonal probe; reacts with azide via click chemistry for biotin tagging. | Conjugating a biotin label to metabolically tagged glycoRNAs for detection and pull-down [55]. |
| Siglec-Fc Chimeras | Soluble, dimeric Siglec receptors; used as probes for ligand binding. | Flow cytometry and immunoprecipitation assays to validate glycoRNA-Siglec binding [1] [56]. |
| NGI-1 | Small-molecule inhibitor of the Oligosaccharyltransferase (OST) complex. | Validating that glycoRNA biogenesis depends on the canonical N-glycosylation machinery [1] [55]. |
| rPAL (RNA-optimized periodate oxidation and aldehyde ligation) | Enrichment/isolation method; targets 1,2-diols in sialic acids. | Identifying the glycan-RNA linkage site; proposed acp3U as a key modified nucleotide [1] [3]. |
| ARPLA (Aptamer & RNA in situ hybridization-mediated proximity ligation assay) | Imaging technology; dual recognition of glycans and RNA. | High-sensitivity visualization of glycoRNAs at the single-cell level [1] [3]. |
Critical Considerations and Emerging Challenges
While the field is advancing rapidly, researchers must be aware of key methodological challenges. Recent studies have highlighted that current glycoRNA isolation protocols can co-purify glycoproteins (such as LAMP1) that are resistant to standard proteinase K digestion under non-denaturing conditions [9] [58]. These glycoproteins can be a source of glycan signals in RNA preparations.
Mitigation Strategies:
- Implement a denaturing proteinase K treatment (e.g., in a buffer containing SDS) during RNA purification to ensure complete protein degradation [9].
- Be aware that the silica column purification step after RNase treatment can cause loss of signal for co-purifying glycoconjugates, which may be misinterpreted as proof of an RNA-specific signal. Controls with exogenous RNA addition can help identify this issue [58].
- Corroborate findings with multiple, orthogonal methods, such as ARPLA imaging and genetic disruption of glycosylation pathways, to build a robust case for true glycoRNA-Siglec interactions.
The functional validation of glycoRNAs as ligands for Siglec receptors establishes a new paradigm in cell surface biology and immune regulation. The experimental frameworks outlined here—combining metabolic labeling, careful biochemical analysis, and specific binding assays—provide a roadmap for researchers to interrogate these interactions. As methods evolve and standards for rigorous validation are adopted, the potential for targeting the glycoRNA-Siglec axis in human diseases, including cancer and inflammatory disorders, represents a compelling frontier for therapeutic development.
Glycosylated RNA (glycoRNA) represents a paradigm-shifting class of biomolecules in which small non-coding RNAs are covalently modified with complex glycans, predominantly localized to the cell surface [10] [6]. This discovery has fundamentally expanded the scope of glycosylation beyond the traditional substrates of proteins and lipids, introducing a previously unrecognized player in cellular communication and immune regulation [3] [5]. The emerging role of glycoRNAs in cancer biology is particularly significant, as these molecules appear to function as critical regulators of tumor progression, immune evasion, and metastatic behavior [3] [2]. Their presence on the cell surface enables direct interaction with immune receptors and other extracellular molecules, positioning them as potential mediators of the tumor microenvironment [3] [59]. This technical review synthesizes the current clinical evidence establishing correlations between glycoRNA expression patterns and cancer malignancy, with a specific focus on metastatic progression across multiple cancer types, and provides detailed methodologies for investigating these relationships in preclinical models.
Quantitative Evidence: GlycoRNA Levels Inversely Correlate with Cancer Progression
Accumulating evidence from multiple cancer models demonstrates a consistent inverse relationship between cell surface glycoRNA abundance and tumor aggressiveness. This pattern has been most comprehensively documented in breast cancer progression series, with extending observations in pancreatic cancer and glioma.
Table 1: GlycoRNA Abundance Across Cancer Progression Models
| Cancer Type | Cell Line / Model | Malignancy Status | GlycoRNA Level | Detection Method | Functional Correlation |
|---|---|---|---|---|---|
| Breast Cancer [3] [60] | MCF-10A | Non-tumorigenic | High | ARPLA | Baseline cellular adhesion |
| MCF-7 | Malignant (Primary) | Medium | ARPLA | Moderate proliferation | |
| MDA-MB-231 | Metastatic | Low | ARPLA | Enhanced invasion & metastasis | |
| Pancreatic Cancer [60] | MIA PaCa-2 | Malignant | Modulated by B4GALT1 | miRNA sequencing | B4GALT1 suppression alters glyco-miRNA, causing cell cycle arrest and apoptosis |
| Glioma [2] | U87, LN229 | Malignant (Primary) | High | Ac4ManNAz labeling, Northern blot | Promotes cell proliferation |
| Patient-derived | Recurrent/Malignant | Variable | Small RNA sequencing | Associated with proliferation capacity |
The breast cancer model provides the most compelling evidence for a direct correlation between glycoRNA loss and metastatic potential. Spatial imaging of glycoRNA in single cells using ARPLA (aptamer and RNA in situ hybridization-mediated proximity ligation assay) has quantitatively demonstrated that non-tumorigenic breast epithelial cells (MCF-10A) exhibit the highest glycoRNA levels, followed by malignant breast cancer cells (MCF-7), with metastatic cells (MDA-MB-231) showing the lowest abundance [3] [60]. This progressive reduction in cell surface glycoRNA suggests that its disappearance may facilitate the acquisition of metastatic capabilities, possibly through altered interactions with the immune system or changes in cell adhesion properties.
In pancreatic cancer, specific glycosylated microRNAs (glyco-miRNAs) have been identified as regulators of oncogenic pathways. Glycosylated miR-103a-3p, miR-122-5p, and miR-4492 have been shown to regulate pancreatic cancer cell growth and proliferation through the PI3K-Akt pathway [60]. Furthermore, the glycosylation enzyme β-1,4-galactosyltransferase 1 (B4GALT1) can suppress the cell cycle and promote apoptosis in MIA PaCa-2 pancreatic cancer cells by affecting the expression of glycol-miRNAs such as hsa-miR-21-5p [60].
Contrary to the pattern observed in breast and pancreatic cancers, glioma cells demonstrate significant enrichment of glycoRNAs [2]. Functional studies in glioma cell lines U87 and LN229 revealed that glycoRNA depletion significantly inhibited glioma cell viability and proliferation, without altering cell adhesion or apoptosis levels [2]. This suggests tissue-specific or context-dependent roles for glycoRNAs in cancer progression, where they may function as promoters of tumor growth in certain malignancies while suppressing metastatic behavior in others.
Table 2: Specific GlycoRNA Species Identified in Cancers
| RNA Species | Cancer Type | Glycan Composition | Functional Role |
|---|---|---|---|
| U2, U4 snRNAs [2] | Glioma | Fucosylated, sialylated complex glycans | Promotion of cell proliferation |
| Y5 RNA [2] | Glioma, various cancers | Sialylated N-glycans | Potential immunomodulation |
| miR-103a-3p, miR-122-5p, miR-4492 [60] | Pancreatic cancer | Undefined | Regulation of PI3K-Akt signaling pathway |
| Multiple snRNAs, snoRNAs, tRNAs [3] | Breast cancer | Sialylated structures | Putative roles in immune recognition |
Molecular Mechanisms: GlycoRNA in Tumor Immune Evasion and Signaling
The functional relationship between glycoRNA expression and cancer progression appears to be mediated through multiple interconnected mechanisms, with immune modulation representing a central pathway.
GlycoRNA-Mediated Immune Evasion
Cell surface glycoRNAs function as ligands for various immune receptors, particularly members of the sialic acid-binding immunoglobulin-like lectin (Siglec) family [3] [6]. These interactions typically transmit inhibitory signals that dampen immune responses against tumor cells. Of the twelve human Siglec receptors, nine have demonstrated binding capacity to cell surface components, with Siglec-11 and Siglec-14 showing particular sensitivity to RNase treatment, suggesting glycoRNA-dependent interactions [3] [6]. In the tumor microenvironment, cancer cells may exploit these interactions to evade immune surveillance: surface glycoRNAs engage Siglec receptors on immune cells, transmitting inhibitory signals that reduce anti-tumor activity and facilitate immune escape [3]. This mechanism represents a promising target for therapeutic intervention, as disrupting these interactions could potentially restore immune recognition of tumor cells.
GlycoRNA Synthesis and Enzymatic Regulation
The biosynthetic pathway of glycoRNA represents a fascinating biological paradox, as it appears to utilize the canonical endoplasmic reticulum-Golgi glycosylation machinery traditionally associated with protein modification, despite the predominant cytoplasmic and nuclear localization of RNA [3] [6]. Key enzymes implicated in glycoRNA formation include N-acetylgalactosaminyltransferases (GALNTs), which may initiate O-glycan addition to RNA, and sialyltransferases that elongate these glycan chains with sialic acids [3]. The oligosaccharyltransferase (OST) complex, essential for N-linked glycosylation of proteins, has also been demonstrated to be required for glycoRNA production [3] [59].
A critical breakthrough in understanding glycoRNA biochemistry came with the identification of 3-(3-amino-3-carboxypropyl)uridine (acp3U) as an RNA modification site that serves as an anchoring point for N-glycan linkage [3]. This modified nucleoside, particularly abundant in tRNAs, provides a molecular platform for glycosylation. Enzymes such as DTW domain-containing 2 (DTWD2) are essential for acp3U formation, and their genetic ablation significantly disrupts glycoRNA biosynthesis and cell surface display [3].
The dysregulation of glycosylation enzymes in cancer cells represents a potential mechanism for altered glycoRNA patterns in tumors. For instance, GALNT14 (influencing O-glycosylation patterns) and ST6GAL1 (adding sialic acid residues to N-glycans) are frequently dysregulated in various cancers and represent promising candidates for mediating cancer-specific glycoRNA modifications [3].
Experimental Protocols: Methodologies for GlycoRNA Detection and Functional Characterization
Metabolic Labeling and Affinity Purification
The foundational protocol for glycoRNA detection utilizes metabolic labeling with peracetylated N-azidoacetylmannosamine (Ac4ManNAz) to introduce a clickable azido-sialic acid into nascent N-glycans [3] [2].
Procedure:
- Cell Culture and Labeling: Culture cancer cells of interest in complete medium supplemented with 100 µM Ac4ManNAz for 24-40 hours [2].
- RNA Extraction: Harvest cells and extract total RNA using TRIzol reagent according to standard protocols [2].
- Size Fractionation: Separate small (<200 nucleotides) and large (>200 nucleotides) RNA fractions using silica columns with adjusted binding buffers [2].
- Click Chemistry: React labeled RNA with DBCO-biotin using strain-promoted azide-alkyne cycloaddition for 2 hours at room temperature [2].
- Affinity Purification: Capture biotinylated glycoRNAs using streptavidin magnetic beads, followed by extensive washing to remove non-specific interactions [2].
- Validation: Analyze purified glycoRNAs by Northern blotting with streptavidin-HRP detection or process for sequencing applications [2].
Critical Controls:
- Include untreated cells (without Ac4ManNAz) as negative controls
- Perform RNase A/T1 treatment to confirm RNA-dependent signals
- Conduct enzymatic digestions with sialidase, PNGase F, and endoglycosidases to verify glycan composition [2]
Spatial Imaging of GlycoRNA (ARPLA)
The ARPLA (sialic acid aptamer and RNA in situ hybridization-mediated proximity ligation assay) technology enables high-sensitivity visualization of glycoRNAs at single-cell resolution [60].
Procedure:
- Cell Preparation: Culture cells on chambered slides under experimental conditions.
- Dual Recognition: Incubate cells simultaneously with:
- Sialic acid-binding aptamers (targeting the glycan moiety)
- Sequence-specific RNA fluorescence in situ hybridization (FISH) probes
- Proximity Ligation: When both probes bind in close proximity (<40 nm), trigger in situ ligation reaction to form circular DNA templates.
- Signal Amplification: Perform rolling circle amplification using phi29 DNA polymerase to generate repetitive sequence arrays.
- Detection: Hybridize fluorescently labeled oligonucleotides to amplified products and visualize by super-resolution microscopy [60].
Applications:
- Quantification of glycoRNA abundance across different cell populations
- Subcellular localization of glycoRNAs
- Correlation of glycoRNA expression with malignant phenotypes
Functional Characterization Through GlycoRNA Depletion
RNA Interference Approach:
- Target Selection: Identify key glycosylation enzymes (GALNTs, sialyltransferases) or RNA-modifying enzymes (DTWD2) for knockdown.
- Knockdown Validation: Transfect cells with siRNA or shRNA constructs and verify target reduction by qRT-PCR and Western blot.
- Phenotypic Assays:
Research Reagent Solutions: Essential Tools for GlycoRNA Investigation
Table 3: Key Research Reagents for GlycoRNA Studies
| Reagent Category | Specific Examples | Function/Application | Considerations |
|---|---|---|---|
| Metabolic Labeling Agents | Ac4ManNAz (N-azidoacetylmannosamine-tetraacylated) | Incorporates azide-modified sialic acid into nascent glycans for bioorthogonal chemistry | Concentration (typically 100 µM) and duration (24-40 hours) require optimization for different cell types [2] |
| Click Chemistry Reagents | DBCO-biotin, BCN-biotin | Strain-promoted azide-alkyne cycloaddition for biotin tagging of labeled glycoconjugates | DBCO derivatives generally offer faster kinetics than azide-alkyne cycloadditions with copper catalysts [2] |
| Affinity Purification Materials | Streptavidin magnetic beads | Capture of biotinylated glycoRNAs from complex RNA extracts | Stringent washing conditions (e.g., high salt, detergent) essential to minimize non-specific binding [2] |
| Enzymatic Tools | RNase A/T1 cocktail, PNGase F, Sialidase, Endo F2/F3 | Specific digestion of RNA or glycan components for validation studies | Enzyme specificity controls crucial; some commercial RNase preparations may contain contaminating nucleases [2] |
| Detection Probes | Sialic acid aptamers, Sequence-specific FISH probes | Dual recognition for ARPLA imaging and related techniques | Optimal probe design requires balancing specificity and accessibility to structured RNA targets [60] |
| Glycosylation Inhibitors | NGI-1 (oligosaccharyltransferase inhibitor) | Disruption of N-linked glycosylation pathways to probe glycoRNA dependence | Cytotoxicity and off-target effects require careful dose-response characterization [3] |
The accumulating clinical evidence firmly establishes that glycoRNA expression patterns correlate significantly with cancer malignancy and metastatic potential across multiple tumor types. The consistent inverse relationship observed in breast cancer models between cell surface glycoRNA abundance and metastatic capability suggests these molecules may function as suppressors of malignant progression, potentially through their interactions with immune receptors such as Siglecs. Conversely, the proliferative role of specific glycoRNAs in glioma indicates tissue-specific functionalities that warrant further investigation.
The translational potential of glycoRNAs encompasses several promising avenues. As biomarkers, their detectable presence on cell surfaces and in extracellular vesicles offers opportunities for non-invasive liquid biopsy applications [60]. Therapeutically, targeting glycoRNA synthesis through inhibition of key enzymes like GALNTs or sialyltransferases represents a novel strategy to disrupt tumor immune evasion mechanisms [3]. Additionally, engineered glycoRNAs could potentially be developed as decoy receptors to modulate immune responses in the tumor microenvironment.
Significant challenges remain in fully elucidating the molecular mechanisms of RNA glycosylation, particularly regarding the subcellular compartmentalization of this process and the precise structural features that determine glycan-RNA linkage specificity. Furthermore, comprehensive profiling of glycoRNA signatures across diverse cancer types and stages will be essential for establishing their clinical utility as diagnostic and prognostic indicators. As methodological advances in detection and imaging continue to enhance our analytical capabilities, glycoRNAs are poised to emerge as significant contributors to our understanding of cancer biology and as promising targets for therapeutic intervention.
Glycosylated RNAs (glycoRNAs) represent a groundbreaking class of biomolecules that challenge long-standing biological dogmas. Traditionally, glycosylation—the enzymatic process of attaching complex sugar chains (glycans)—was considered the exclusive domain of proteins and lipids. Similarly, RNA was largely viewed as an intracellular molecule. The discovery that small non-coding RNAs can be covalently modified with N-glycans and displayed on the cell surface has fundamentally reshaped our understanding of both glycobiology and RNA biology [1] [43]. These glycoRNAs are predominantly modified with sialylated and fucosylated N-glycans and are surprisingly abundant on the extracellular surface of mammalian cells, positioning them to play direct roles in intercellular communication, particularly with the immune system [1] [8] [24]. This whitepaper synthesizes current research on glycoRNAs, with a specific focus on their emerging potential as novel biomarkers for disease detection and as tractable targets for therapeutic intervention in cancer and inflammatory diseases.
GlycoRNA Fundamentals: Composition, Biosynthesis, and Localization
Molecular Identity and Structural Insights
GlycoRNAs are defined by a unique hybrid structure. Their RNA backbone primarily consists of small non-coding RNAs, including small nuclear RNAs (snRNAs like U2 and U4), Y RNAs, transfer RNAs (tRNAs), and microRNAs (miRNAs) [1] [2] [24]. Crucially, the glycan component is not a simple monosaccharide but a complex N-glycan, rich in sialic acid and fucose, which is structurally related to, yet distinct from, the N-glycans found on glycoproteins [1] [24]. The covalent linkage between the RNA and glycan moieties has been identified as occurring through a specific modified nucleotide, 3-(3-amino-3-carboxypropyl)uridine (acp3U), which serves as the anchoring site for the glycan chain [1] [52].
Biosynthetic Pathway and Surface Display
The biosynthesis of glycoRNAs is an area of active investigation, but current evidence points to a non-canonical pathway that bridges intracellular and secretory compartments. A proposed model suggests a multi-step process:
- acp3U Modification: The acp3U base is introduced into specific tRNAs and other small RNAs during their maturation in the nucleus and cytosol [24].
- Glycan Attachment: The RNA molecule enters the secretory pathway, where the classical endoplasmic reticulum-Golgi N-glycosylation machinery, including the oligosaccharyltransferase (OST) complex, facilitates the attachment of sialylated N-glycans [1] [24].
- Trafficking and Display: The mature glycoRNAs are transported and displayed on the outer leaflet of the plasma membrane, where they can interact with extracellular binding partners [1] [52]. Their surface localization is further organized through interactions with cell-surface RNA-binding proteins (csRBPs) such as nucleolin and HNRNPU, forming specialized nanoscale domains or clusters that are critical for their function [1] [52].
The following diagram illustrates the proposed biosynthetic pathway and functional display of glycoRNAs on the cell surface.
Therapeutic Potential in Human Disease
GlycoRNAs as Novel Biomarkers
The presence of glycoRNAs on the cell surface and in small extracellular vesicles (sEVs) makes them exceptionally accessible for liquid biopsy and diagnostic applications. Their dysregulated expression in specific disease states provides a foundation for their use as biomarkers.
Table 1: GlycoRNA Dysregulation in Disease Models
| Disease Model | Key Findings on GlycoRNA Dysregulation | Potential Diagnostic/Therapeutic Implication |
|---|---|---|
| Glioma | GlycoRNAs (especially U2, U4 snRNAs) are highly abundant on glioma cells (U87, LN229 lines); depletion inhibits cell viability and proliferation [2]. | Potential biomarker for brain tumors; novel target for anti-proliferative therapy. |
| Acute Myeloid Leukemia (AML) | GlycoRNAs form nanoclusters with RNA-binding proteins on leukemia cells; removal enhances immune cell-mediated cancer cell killing [8]. | Target for disrupting immune evasion mechanisms. |
| General Cancer Diagnostics | GlycoRNAs on sEVs from clinical serum samples can distinguish cancer vs. control with high accuracy (~100%) and subclassify cancer types (~90% accuracy) [8] [24]. | Powerful, non-invasive biomarker for early cancer detection and classification. |
The diagnostic power of glycoRNAs is being unlocked by advanced detection technologies. For instance, the dual-recognition FRET (drFRET) imaging technology enables ultrasensitive visualization of glycoRNAs on sEVs from minute volumes (e.g., 10 µL) of patient biofluids like serum, demonstrating remarkable diagnostic precision [8] [24].
GlycoRNAs as Drug Targets in Cancer and Inflammation
GlycoRNAs are emerging as functionally significant mediators in disease pathogenesis, making them attractive targets for therapeutic intervention.
- *Cancer Immune Evasion:* On cancer cells, surface-displayed glycoRNAs can engage with immunoregulatory receptors such as Siglec-10 and Siglec-11 [1] [8]. These interactions are hypothesized to deliver inhibitory signals to immune cells, akin to established immune checkpoints like PD-1, thereby facilitating tumor immune evasion. Therapeutic strategies aimed at blocking these specific glycoRNA-Siglec interactions could reinvigorate anti-tumor immunity [1] [8].
- *Inflammatory Cell Trafficking:* GlycoRNAs on immune cells, such as neutrophils and monocytes, play a critical role in their recruitment to sites of inflammation. Studies show that glycoRNAs mediate adhesion to endothelial cells through interactions with P-selectin [1] [8]. Furthermore, macrophage activation by inflammatory stimuli like lipopolysaccharide (LPS) leads to a marked increase in surface glycoRNA abundance, and their removal impairs cellular adhesion [8]. This positions glycoRNAs as promising targets for modulating aberrant inflammatory responses in autoimmune and other inflammatory diseases [61] [46].
The diagram below summarizes the key signaling pathways through which glycoRNAs influence cancer and inflammatory diseases.
The Scientist's Toolkit: Key Reagents and Methodologies
Research into glycoRNAs relies on a specialized set of reagents and protocols designed to detect and characterize these low-abundance, hybrid molecules.
Table 2: Essential Research Reagent Solutions for GlycoRNA Studies
| Research Reagent / Tool | Core Function | Key Application in GlycoRNA Research |
|---|---|---|
| Ac4ManNAz (Peracetylated N-azidoacetylmannosamine) | A metabolic precursor that incorporates "clickable" azide groups into nascent sialic acids of glycans [2] [9]. | Metabolic labeling of glycoRNAs for subsequent conjugation to biotin or fluorescent probes via click chemistry (e.g., for Northwestern blot) [2]. |
| RNA-optimized Periodate Oxidation and Aldehyde Ligation (rPAL) | A chemical method that oxidizes 1,2-diols on sialic acids to create aldehydes for specific tagging and enrichment [1]. | Highly sensitive (~25-fold vs. metabolic labeling) enrichment and isolation of native glycoRNAs for downstream analysis [1] [24]. |
| Sialidase (Neuraminidase) & PNGase F | Glycan-cleaving enzymes; Sialidase removes terminal sialic acid, PNGase F cleaves entire N-glycans from substrates [2]. | Confirming the glycan-dependent nature of signals; used as negative controls in detection assays [2]. |
| Aptamer and RNA In Situ Hybridization-mediated Proximity Ligation Assay (ARPLA) | A dual-recognition technique using a sialic acid aptamer and RNA-FISH probes to trigger a localized amplification reaction [1]. | High-sensitivity and single-cell resolution visualization of spatial distribution of glycoRNAs [1] [8]. |
| Dual-recognition FRET (drFRET) | A dual-probe system where one probe targets the glycan and another the RNA, generating a FRET signal only when both are in close proximity [1] [24]. | Ultrasensitive detection and profiling of glycoRNAs on extracellular vesicles (sEVs) from clinical biofluid samples for diagnostic purposes [1] [24]. |
Detailed Experimental Protocol: Metabolic Labeling and Northwestern Blot
This protocol is a cornerstone for the initial detection and validation of glycoRNAs [2] [24].
- Metabolic Labeling: Culture cells (e.g., glioma cell lines U87, LN229) in medium supplemented with 100 µM Ac4ManNAz for a defined period (e.g., 24-40 hours) to allow for incorporation of the azide-modified sialic acid into glycoRNAs.
- RNA Extraction: Lyse cells and extract total RNA using a standard TRIzol-based protocol.
- Click Chemistry Biotinylation: React the extracted RNA with DBCO-Biotin (a cyclooctyne-conjugated biotin) via strain-promoted azide-alkyne cycloaddition (SPAAC). This covalently links biotin specifically to the azide-labeled glycans on glycoRNAs.
- RNA Purification: Purify the biotinylated RNA using silica column-based purification (e.g., Zymo Spin columns) to remove unreacted click chemistry reagents.
- Electrophoresis and Blotting: Separate the RNA samples by denaturing gel electrophoresis and transfer to a membrane.
- Signal Detection: Probe the membrane with Streptavidin-HRP conjugate and develop using a chemiluminescent substrate. The presence of biotin signals confirms the existence of glycosylated RNA species. Specificity controls are critical and should include:
- No Ac4ManNAz control (0 h group): To ensure signals are dependent on metabolic labeling.
- RNase A/T1 treatment: To confirm the signal is derived from an RNA molecule.
- Sialidase/PNGase F treatment: To confirm the signal is dependent on the glycan structure.
Challenges and Future Directions
Despite rapid progress, the field of glycoRNA biology faces several fundamental challenges that must be addressed to fully realize its therapeutic potential. A primary concern involves ongoing discussions about potential artifacts in purification protocols, with some studies suggesting that glycoproteins can co-purify with small RNA preparations and may contribute to glycan signals [9]. This underscores the need for rigorous controls and continued refinement of isolation methods. Other key unresolved questions include the complete elucidation of the biosynthetic pathway, particularly the mechanism by which RNA substrates access the glycosylation machinery; the full structural diversity of glycoRNA glycans; and a deeper understanding of their precise functions in vivo [8] [9].
Future research will focus on leveraging advanced tools like rPAL and ARPLA to map the "glycoRNAome" across different tissues and disease states. Therapeutically, the most promising near-term directions include the development of blocking antibodies or engineered decoy receptors to inhibit pathogenic glycoRNA-Siglec interactions in cancer and autoimmune diseases, and the continued exploration of extracellular vesicle glycoRNAs as a rich source of non-invasive biomarkers for early disease detection and monitoring [8] [24].
GlycoRNA, a recently discovered class of macromolecules comprising small non-coding RNAs modified with sialylated glycans, has emerged as a significant contributor to immune system regulation. Discovered in 2021, glycoRNAs challenge traditional biological paradigms by representing a third scaffold for glycosylation beyond proteins and lipids [6] [28]. These molecules are primarily displayed on the outer surface of the plasma membrane, where they participate in critical intercellular communication processes [6] [1]. Their position at the cell-environment interface enables direct interaction with various immune receptors, positioning glycoRNAs as potent modulators of immune responses, including neutrophil recruitment and autoimmune mechanisms [14] [62] [60]. This whitepaper examines the technical evidence establishing glycoRNA's role in these specific immune processes, providing researchers with current methodologies, mechanistic insights, and experimental frameworks for advancing this emerging field.
GlycoRNA in Autoimmunity: Molecular Mechanisms and Ligand Interactions
The involvement of glycoRNA in autoimmunity stems from its dual identity as both a glycan and an RNA molecule—two elements with established roles in self-recognition and immune tolerance. A key mechanism involves its function as a ligand for Siglec (Sialic acid-binding immunoglobulin-like lectin) receptors, which are immunoregulatory receptors on immune cells [6] [1].
Siglec Receptor Engagement
Direct Binding Evidence: Systematic screening using soluble Siglec-Fc reagents demonstrated that 9 out of 12 human Siglecs bind to HeLa cells, with Siglec-11 and Siglec-14 binding being particularly sensitive to RNase A treatment [6]. This RNase sensitivity confirms RNA's essential role in these interactions and suggests glycoRNAs may serve as native ligands for these orphan receptors.
Immune Checkpoint Implications: Siglec receptors are increasingly recognized as important immune checkpoints, similar to PD-1 and CTLA-4, making their glycoRNA ligands potentially significant for therapeutic modulation [1] [3]. The interaction between cell surface glycoRNAs and Siglecs may transmit inhibitory signals that contribute to immune evasion mechanisms, particularly in cancer contexts [3].
Autoantibody Recognition
GlycoRNAs also interact with anti-double-stranded RNA antibodies, which are typically associated with RNA virus-infected cells but also appear in autoimmune conditions like systemic lupus erythematosus (SLE) [6] [3]. This suggests glycoRNAs may contribute to the generation of autoantigens in rheumatic diseases by exposing RNA epitopes in an immunogenic context on the cell surface.
Table 1: Experimental Evidence for GlycoRNA in Autoimmune Contexts
| Evidence Type | Experimental System | Key Finding | Technical Approach |
|---|---|---|---|
| Receptor Binding | HeLa cells; Siglec-Fc reagents | 9/12 human Siglecs bind cells; Siglec-11 & -14 binding is RNase-sensitive [6] | Soluble receptor binding assays with RNase controls |
| Ligand Identification | Multiple cell lines | GlycoRNAs are potential native ligands for Siglec family members [6] [1] | Flow cytometry with Siglec-Fc chimeras |
| Autoantigen Potential | Cell surface mapping | GlycoRNAs bind anti-dsRNA antibodies used to detect viral infection [6] | Immunoassays with autoimmune sera |
| Therapeutic Relevance | Cancer-immune evasion models | GlycoRNA-Siglec interactions may transmit inhibitory signals to immune cells [3] | Functional immune cell assays |
GlycoRNA-Mediated Neutrophil Recruitment: Mechanisms and Functional Significance
A 2024 study provided compelling evidence establishing glycoRNA's critical role in neutrophil recruitment, offering the first clear functional demonstration of its physiological importance [62] [60].
P-Selectin Recognition
The study identified that neutrophil surface glycoRNAs serve as ligands for P-selectin (Selp) on endothelial cells [62] [60]. This interaction facilitates the initial tethering and rolling of neutrophils along the vascular endothelium—the essential first step in the leukocyte adhesion cascade that precedes transmigration into inflamed tissues.
SIDT Transporter Dependence
The expression and surface display of glycoRNAs in neutrophils depend on mammalian homologs of the sid-1 RNA transporter, specifically SIDT1 and SIDT2 [62]. Genetic knockdown of these transporters ablates neutrophil glycoRNAs and functionally impairs recruitment, confirming their necessity in the process.
Functional Validation
In Vivo Models: In murine models of peritonitis, elimination of cell surface RNAs substantially impaired neutrophil recruitment to inflammatory sites [62]. The disruption of glycoRNA expression via Sidt gene knockdown mimicked this phenotype, reducing neutrophil infiltration.
In Vitro Assays: Neutrophils lacking surface RNAs showed reduced adhesion to and migration through endothelial cell monolayers, establishing the importance of these molecules in the sequential steps of diapedesis [62].
Table 2: Quantitative Effects of GlycoRNA Disruption on Neutrophil Function
| Functional Parameter | Experimental Condition | Observed Effect | Biological Significance |
|---|---|---|---|
| In Vivo Recruitment | Sidt gene knockdown in murine peritonitis | Substantially impaired neutrophil recruitment to inflammatory sites [62] | Confirms essential role in physiological inflammation |
| Adhesion to Endothelium | Elimination of surface RNAs | Reduced neutrophil adhesion to endothelial cells [62] | Disrupts initial contact essential for extravasation |
| Transendothelial Migration | Elimination of surface RNAs | Impaired migration through endothelial barriers [62] | Blocks critical step in reaching inflamed tissues |
| Ligand Specificity | Comparison of P- vs. E-selectin binding | P-selectin (Selp) specifically recognizes glycoRNAs [60] | Reveals unique specificity among highly similar lectins |
Experimental Methodologies for GlycoRNA Research
Detection and Imaging Platforms
ARPLA (Aptamer and RNA in situ hybridization-mediated Proximity Ligation Assay): This method enables high-sensitivity visualization of glycoRNAs at the single-cell level through dual recognition of glycans and RNA sequences [1] [60]. The technique employs:
- Glycan-specific aptamers (e.g., sialic acid binders)
- Sequence-specific RNA FISH probes
- Proximity-dependent ligation and rolling circle amplification
- Fluorescently labeled oligonucleotides for signal output
drFRET (Dual-recognition FRET): This technology enables visualization of glycosylated RNAs in small extracellular vesicles from cancer cell lines and clinical serum samples [1]. It facilitates the study of glycoRNA interactions with Siglec-10, Siglec-11, and P-selectin in native contexts.
rPAL (RNA-optimized Periodate oxidation and Aldehyde Ligation): A chemical biology approach that leverages periodate oxidation of 1,2-diols in sialic acids to generate aldehydes, which then form stable oxime bonds with aminooxy-functionalized solid-phase supports [1]. This method allows specific enrichment, isolation, and characterization of glycoRNAs.
Functional Analysis Workflows
Genetic/Pharmacological Inhibition: Studies have employed inhibition of oligosaccharyltransferase (OST) and key glycan biosynthetic machinery to establish the dependence of glycoRNA production on conventional glycosylation pathways [6] [1].
Biochemical Fractionation: Subcellular fractionation followed by RNA extraction has demonstrated glycoRNA's association with the outer leaflet of cellular membranes [6] [1].
Receptor Binding Assays: Soluble Siglec-Fc and Selectin-Fc chimeras combined with flow cytometry have been instrumental in establishing specific interactions with immune receptors [6] [62].
Diagram 1: GlycoRNA mediates neutrophil recruitment via P-selectin binding.
Research Reagent Solutions and Technical Tools
Table 3: Essential Research Reagents for GlycoRNA Investigation
| Reagent/Tool | Primary Function | Application Context | Key Characteristics |
|---|---|---|---|
| Metabolic Labels (Ac4ManNAz) | Precursor for clickable azido-sialic acid in nascent N-glycans [9] | Metabolic labeling and pull-down assays | Enables bioorthogonal chemistry via azide group |
| ARPLA Reagents | Dual recognition of glycans and RNA for spatial imaging [1] [60] | Single-cell glycoRNA visualization | High sensitivity and selectivity; works in fixed cells |
| rPAL Chemistry | Periodate-based enrichment via sialic acid diol oxidation [1] | GlycoRNA isolation and characterization | Specific for glycoRNA; compatible with mass spec |
| Siglec-Fc Chimeras | Soluble receptors for binding assays [6] | Receptor-ligand interaction studies | 12 human Siglecs available; Fc portion enables detection |
| SIDT1/SIDT2 KO/KD Systems | Genetic ablation of glycoRNA transport [62] | Functional validation studies | Critical for establishing neutrophil recruitment mechanisms |
| Lectin Arrays | Profiling of glycan presentations on RNA [63] | GlycoRNA capture and analysis | Various lectins with different sugar specificities |
| Proteinase K (Denaturing) | Control for glycoprotein contamination [9] | Method validation | Distinguishes true glycoRNA from co-purifying proteins |
Diagram 2: Experimental workflow for glycoRNA analysis.
Technical Considerations and Controversies
Recent investigations have highlighted critical methodological considerations in glycoRNA research. A 2025 study demonstrated that glycoproteins—particularly LAMP1—can co-purify with small RNA preparations using current protocols, representing a potential source of contaminating glycans [9]. These glycosylated molecules showed resistance to RNase A/T1 but sensitivity to proteinase K digestion under denaturing conditions [9]. This emphasizes the necessity for:
- Rigorous enzymatic controls including proteinase K with denaturation
- Orthogonal validation methods
- Careful interpretation of glycan signals in RNA preparations
- Development of more specific isolation protocols
The field has responded with improved methodological frameworks, including specialized databases like GlycoRNAdb which integrates experimentally supported glycoRNA sequences with associated glycosylation sites and expression profiles [16].
GlycoRNA represents a transformative discovery in molecular immunology, with established roles in neutrophil recruitment and strong implications for autoimmune disease mechanisms. The interaction between glycoRNAs and specific immune receptors (Siglecs and P-selectin) provides a molecular framework for understanding how these novel biomolecules influence inflammatory processes. For researchers and drug development professionals, targeting glycoRNA synthesis or its receptor interactions offers promising therapeutic avenues for modulating immune responses in autoimmunity, cancer, and other inflammatory conditions. Future research should focus on elucidating the precise biosynthetic pathway, expanding the repertoire of validated receptor interactions, and developing more specific tools to probe glycoRNA function in physiological and disease contexts.
Conclusion
The discovery of glycoRNA has fundamentally expanded our understanding of molecular biology, establishing a new class of biomolecules at the intersection of RNA biology and glycobiology. Key takeaways confirm their identity as authentic, surface-displayed molecules with critical roles in immune system modulation, particularly through interactions with Siglec receptors. Advanced methodologies like rPAL, ARPLA, and drFRET are enabling sensitive detection and spatial mapping, revealing their promising diagnostic potential, especially in oncology. Future research must focus on fully elucidating the biosynthetic pathway, defining the complete 'glycoRNAome,' and exploiting these molecules for therapeutic intervention. For biomedical and clinical research, glycoRNAs represent a frontier with immense potential for developing novel diagnostic platforms, targeted cancer therapies, and modulators of immune function, heralding a new era in precision medicine.
References
- 1. GlycoRNA, a novel RNA modification - PMC - PubMed Central [https://pmc.ncbi.nlm.nih.gov/articles/PMC12494505/]
- 2. GlycoRNAs are abundant in glioma and involved in ... [https://www.nature.com/articles/s41389-025-00570-5]
- 3. GlycoRNA: A new player in cellular communication - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC12034019/]
- 4. Small RNAs are modified with N-glycans and ... - PubMed [https://pubmed.ncbi.nlm.nih.gov/34004145/]
- 5. Unveiling GlycoRNA: New Study Proves They Do Exist | HSCRB [https://hscrb.harvard.edu/news/unveiling-glycorna-flynn/]
- 6. GlycoRNA [https://en.wikipedia.org/wiki/GlycoRNA]
- 7. Glycan-RNA: a new class of non-coding RNA [https://bio-integration.org/10-15212-bioi-2021-0032/]
- 8. Forum GlycoRNAs as emerging drug targets [https://www.sciencedirect.com/science/article/abs/pii/S0165614725001725]
- 9. Proteins are a source of glycans found in preparations ... [https://www.nature.com/articles/s12276-025-01575-1]
- 10. GlycoRNA research: from unknown unknowns to known ... [https://pubmed.ncbi.nlm.nih.gov/41264770/]
- 11. The pathways of secretory cargo export at the endoplasmic ... [https://www.nature.com/articles/s41467-025-57408-2]
- 12. Unveiling Sec31: The regulator of COPII vesicle-dependent ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12529364/]
- 13. Quantitative intra-Golgi transport and organization data ... [https://elifesciences.org/articles/98582]
- 14. The emerging role of GlycoRNAs in immune regulation and ... [https://www.sciencedirect.com/science/article/pii/S0165247825000811]
- 15. Quantitative imaging of lipid transport in mammalian cells [https://www.nature.com/articles/s41586-025-09432-x]
- 16. GlycoRNAdb: a database of glycoRNA sequences ... [https://pubmed.ncbi.nlm.nih.gov/41273091/]
- 17. acp3U, an amino-acid-containing nucleoside, links N-glycans ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11533151/]
- 18. a uridine RNA modification drives glycoRNA biogenesis [https://www.nature.com/articles/s41392-024-02056-z]
- 19. Certain RNA modifications could contribute to the ... [https://www.eurekalert.org/news-releases/1093256]
- 20. Exploring Foundational Experiments in GlycoRNA [https://www.preprints.org/manuscript/202411.0096/v1]
- 21. FRET imaging of glycoRNA on small extracellular vesicles ... [https://www.nature.com/articles/s41467-025-58490-2]
- 22. Protocol for detecting glycoRNAs using metabolic labeling ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11426122/]
- 23. Clier-qPCR:Quantitative Validation of GlycoRNAs [https://www.creative-biolabs.com/anti-glycan-antibodies/clier-qpcr-for-glycorna-analysis.htm]
- 24. Introducing GlycoRNAs, a new frontier in small RNA biology. [https://www.revvity.com/blog/introducing-glycornas-new-frontier-small-rna-biology]
- 25. What Are GlycoRNAs? [https://www.arraystar.com/reviews/what-are-glycornas/]
- 26. FRET imaging of glycoRNA on small extracellular vesicles ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11985951/]
- 27. How to Study GlycoRNAs? [https://www.arraystar.com/reviews/how-to-study-glycornas/]
- 28. GlycoRNA Insights: Latest Research and Applications [https://www.creative-biolabs.com/anti-glycan-antibodies/glycorna-insights.htm]
- 29. Spatial imaging of glycoRNA in single cells with ARPLA [https://pmc.ncbi.nlm.nih.gov/articles/PMC10663388/]
- 30. a revolutionary glycoRNA imaging tool with precision and power [http://utotc.technologypublisher.com/technology/54752]
- 31. ARPLA for spatial imaging of glycoRNAs [https://www.nature.com/articles/s41568-023-00614-1]
- 32. A practical guide for choosing an optimal spatial ... [https://bmcgenomics.biomedcentral.com/articles/10.1186/s12864-025-11235-3]
- 33. Transcriptome-wide identification of glycoRNAs by Clier- ... [https://link.springer.com/article/10.1007/s11427-024-2989-x]
- 34. GlycoRNA-seq: High-Throughput Sequencing of ... [https://www.creative-biolabs.com/anti-glycan-antibodies/glycorna-seq-for-glycorna-analysis.htm]
- 35. Transcriptome-wide identification of glycoRNAs by Clier- ... [https://pubmed.ncbi.nlm.nih.gov/41177858/]
- 36. GlycoRNA Sequencing Service: Map Glycosylated RNAs ... [https://www.creative-proteomics.com/ptms-proteomics/glycorna-sequencing.htm]
- 37. RNA-binding proteins and glycoRNAs form domains on the ... [https://www.sciencedirect.com/science/article/abs/pii/S0092867425001096]
- 38. Biology of extracellular vesicles and the potential of tumor- ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11759217/]
- 39. Extracellular Vesicles as Diagnostic Biomarkers in Cancer [https://www.trillium.de/en/journals/trillium-extracellular-vesicles/archive/2025/trillium-extracellular-vesicles-2025/review/ev-diagnostics-extracellular-vesicles-as-diagnostic-biomarkers-in-cancer-from-technological-advances-to-clinical-translation.html]
- 40. Professor Xu Zhangrun's Team from NEU Published a New ... [https://english.neu.edu.cn/info/1003/14581.htm]
- 41. Glycosylation: mechanisms, biological functions and ... [https://www.nature.com/articles/s41392-024-01886-1]
- 42. Structural and mechanistic studies of the N-glycosylation ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10859629/]
- 43. Discovery of glycoRNAs upends cell biology [https://answers.childrenshospital.org/glycorna/]
- 44. Tools to investigate the cell surface: proximity as a central ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11193615/]
- 45. Latest Trends in Synthetic Glycan Chemistry (2025 Outlook) [https://www.bocsci.com/glycan/resources/latest-trends-in-synthetic-glycan-chemistry-2025-outlook.html?srsltid=AfmBOoo18au3mTcD2gnJT5qUIwZIfHSYb27KniPXliPG6GWB6onYkRNO]
- 46. The emerging role of GlycoRNAs in immune regulation and ... [https://pubmed.ncbi.nlm.nih.gov/40451487/]
- 47. : From Biomodulation to Immunotherapy Siglecs [https://pubmed.ncbi.nlm.nih.gov/40605153/]
- 48. Insights on preventing organ transplant rejection | ScienceDaily [https://www.sciencedaily.com/releases/2025/05/250507141142.htm]
- 49. -mediated Siglec of regulation ... | Nature Reviews Immunology immune [https://www.nature.com/articles/nri3737?error=cookies_not_supported]
- 50. Frontiers | Elevated Siglec -7 expression correlates with adverse... [https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2025.1573365/full]
- 51. GlycoRNA-seq Services [https://www.cd-genomics.com/epigenetics/glycorna-seq.html]
- 52. Cell Surface glycoRNA: A New Role That Subverts ... [https://www.bioglyco.com/blog/cell-surface-glycorna-a-new-role-that-subverts-traditional-biology.html]
- 53. Small RNAs are modified with N-glycans and displayed on the ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9097497/]
- 54. The modified RNA base acp3U is an attachment site for N- ... [https://www.sciencedirect.com/science/article/pii/S0092867424008389]
- 55. GlycoRNA-L and glycoRNA-S mediate human monocyte ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12281301/]
- 56. Siglec Ligands - PMC - PubMed Central - NIH [https://pmc.ncbi.nlm.nih.gov/articles/PMC8161119/]
- 57. Siglecs and their roles in the immune system [https://www.nature.com/articles/nri2056]
- 58. RNA-associated glycoconjugates highlight potential ... [https://www.nature.com/articles/s12276-025-01585-z]
- 59. GlycoRNA, a novel RNA modification | Discover Oncology [https://link.springer.com/article/10.1007/s12672-025-03660-3]
- 60. Why Study GlycoRNAs? [https://www.arraystar.com/reviews/why-study-glycornas/]
- 61. GlycoRNAs as emerging drug targets [https://pubmed.ncbi.nlm.nih.gov/40849276/]
- 62. Cell surface RNAs control neutrophil recruitment - PubMed - NIH [https://pubmed.ncbi.nlm.nih.gov/38262409/]
- 63. GlycoRNA Array | Profile glycosylated RNA [https://www.arraystar.com/glycorna-array-service/?print=y]